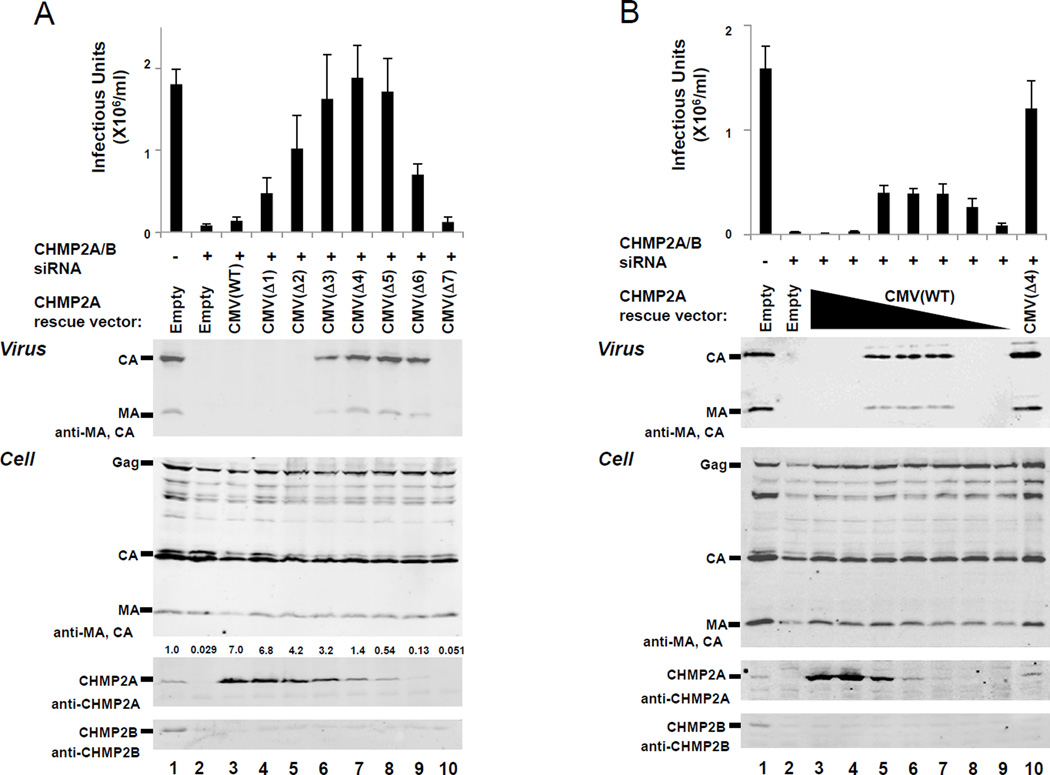
Figure 2. Rescue of HIV-1 budding from 293T cells that lack endogenous CHMP2 proteins by expression of human CHMP2A from attenuated CMV expression vectors.
(A) Differential rescue of HIV-1 budding by CHMP2A proteins expressed from the ensemble of different pCMV-CHMP2A expression vectors. HIV-1 vector infectivity titers (top panel) and western blots showing protein levels in culture supernatants (Virus, panel 2) or 293T cells (Cell, panels 3–5) co-transfected with: a proviral HIV-1 vector (500 ng of pCMV-dR8.2, 500 ng pLox-GFP, 250 ng pMD-G)(25) (all lanes), either 20 nM control siRNA duplex (CGUACGCGGAAUACUUCGAtt, where “tt” represents two overhanging deoxyribothymidines, lanes 1) or 10 nM each of siRNA duplexes against CHMP2A and CHMP2B (AGGCAGAGAUCAUGGAUAUtt and GGAACAGAAUCGAGAGUUAtt, lanes 2–10)(15), and 500 ng of either an empty vector control (lane 2) or the designated pCMV-CHMP2A vector expressing an siRNA-resistant CHMP2A construct (lanes 3–10). Integrated CHMP2A band intensities, normalized to the endogenous CHMP2A level, are provided over each lane in panel 4. 293T cells (2×105 cells/well, 6-well plates, 2 ml volume) were seeded at t=0, transfected with siRNA (20 nM final total concentration, 7.5 µl Lipofectamine RNAiMAX; Life Technologies, Carlsbad, California) at t=24h, and co-transfected with siRNA, the designated pCMV-CHMP2A vector (500 ng), and the HIV-1 vector (20nM final total siRNA concentration, 500 ng pCMV-dR8.2, 500 ng pLox-GFP, 250 ng pMD-G, 10 µl Lipofectamine 2000; Life Technologies) at t=48h. The following silent mutations were introduced into the CHMP2A cDNA coding sequence to make the CHMP2A mRNA siRNA resistant: AGGCAGAGATCATGGATAT to AaGCtGAaATtATGGATAT (nucleotides 395–413). Cells and supernatant were collected and analyzed at t=96h. Released virions were pelleted through a 20% sucrose cushion at 15,000 × g and viral Gag-derived proteins were detected by western blotting using our rabbit anti-HIV-1 CA (UT415, 1:2000) and MA (UT556, 1:1000) antisera. Cells were lysed with buffer (50mM Tris-HCl pH 7.4, 150mM NaCl, 1% Triton-X100 and PMSF) for western blotting of intracellular proteins. Anti-CHMP2A and CHMP2B were detected with UT589 (our antibody) and Ab33174 (Abcam, Cambridge, MA) as described (26). Secondary antibodies were anti-mouse IgG or anti-rabbit IgG polyclonal conjugated to IRdye700 or IRdye800 (1:10000, Rockland Immunochemicals Inc., Gilbertsville, PA). Western blots were visualized using an Odyssey scanner (Li-Cor Biosciences, Lincoln, NB). For titer measurements, 293T cells were infected with viral supernatants and GFP-positive cells were quantified by flow cytometry (FL1 channel, FACScan, BD). Values show the average of three independent repetitions with standard errors.
(B) Rescue of HIV-1 budding by CHMP2A proteins expressed from different quantities of the wild type CMV expression vector, pCMV(WT)-CHMP2A. The figure and experiments are equivalent to panel (A) except that the following quantities of the siRNA-resistant CHMP2A rescue construct, pCMV(WT)-CHMP2A, were transfected: 500 ng (lane 3), 170 ng (lane 4), 56 ng (lane 5), 19 ng (lane 6), 6.2 ng (lane 7), 2.1 ng lane 8), and 0.69 ng (lane 9). In the experiments shown in lanes 4–9, total expression vector levels were adjusted to 500 ng with pCMV(WT) empty vector. The sample shown in Lane 10 was transfected with 500 ng of the pCMV(Δ4)-CHMP2A expression vector positive control).