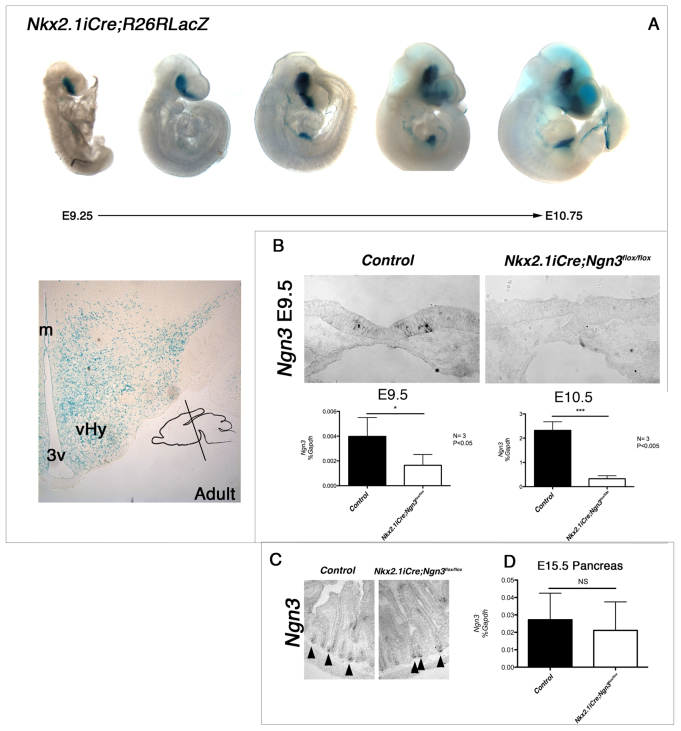
Fig. 1.
Nkx2.1iCre drives Ngn3 deletion in the ventral forebrain. (A) Expression of Nkx2.1iCre in the developing mouse embryo. R26RLacZ reporter females were crossed with Nkx2.1iCre males, and resulting embryos stained with X-Gal to reveal lacZ reporter expression. β-gal-positive cells are observed in the ventral forebrain as early as E9.25 and throughout the adult hypothalamus. β-gal-positive cells are also observed in endodermal-derived tissues from around E10.0. Insert represents angle of section throughout study. m, midline; 3v, third ventricle; vHy, ventral hypothalamus. (B) Ngn3 was conditionally deleted in Nkx2.1-expressing cells by crossing Nkx2.1iCre males with Ngn3flox/flox females, resulting in Ngn3 conditional mutant mice (Nkx2.1iCre/+;Ngn3flox/flox). Following deletion, Ngn3 expression was not observable by in situ hybridization at E9.5. However, qRT-PCR reveals low levels of Ngn3 in the E9.5 ventral forebrain, whereas only residual levels are seen at E10.5. Student’s t-test: *P<0.05 and ***P<0.005. (C,D) Despite iCre-positive cells being observed in the developing endoderm, no change was seen in the expression of Ngn3 by in situ hybridization in the adult intestinal crypt cells (C), nor by qRT-PCR in the developing pancreas (D). Arrowheads in C indicate Ngn3-positive crypt cells. NS, not significant.