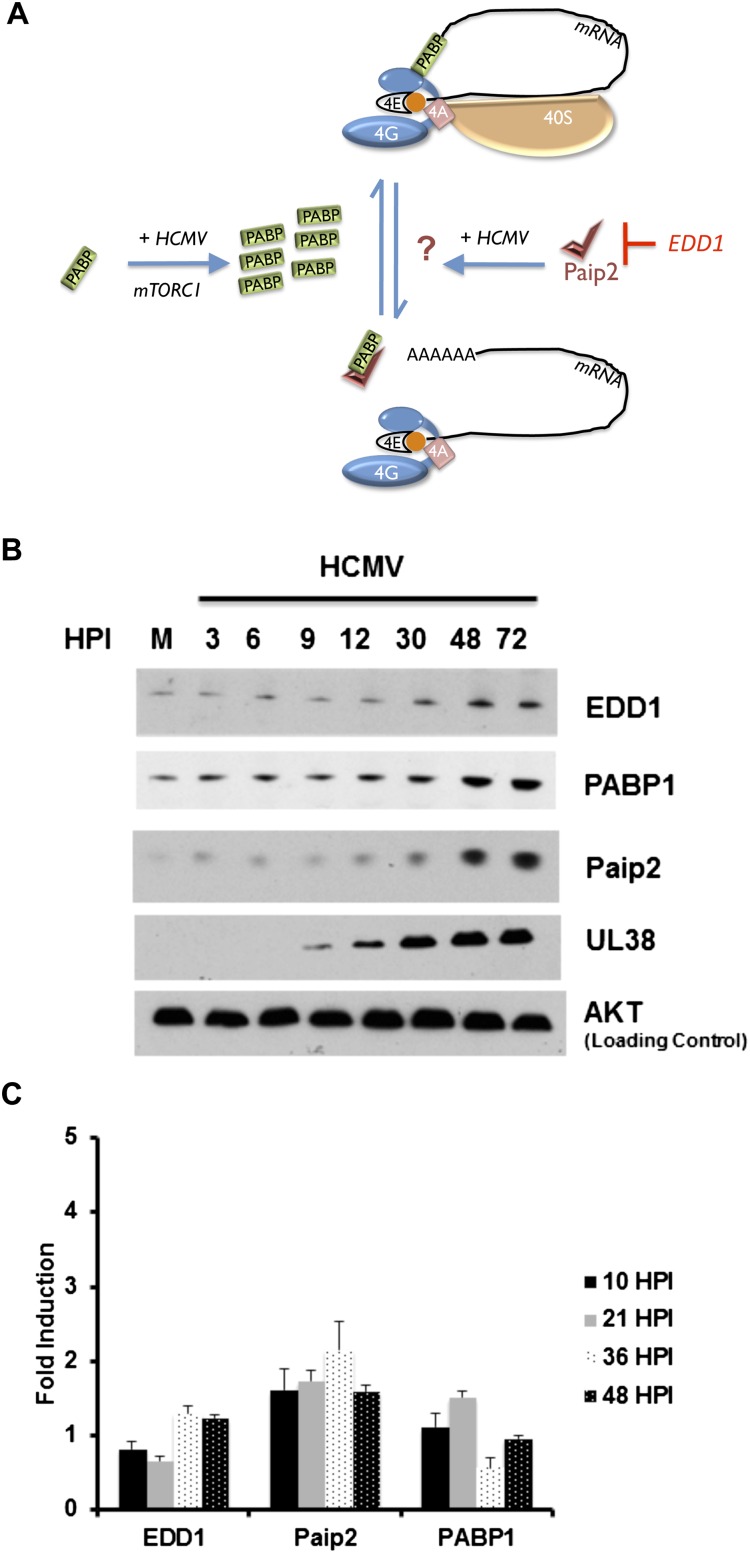
Figure 1.
Accumulation of the cellular PABP repressor Paip2; its negative regulator, EDD1; and PABP1 in HCMV-infected cells. (A) Cartoon illustrating control of PABP homeostasis in response to serum stimulation or HCMV infection. PABP is encoded by an mRNA containing a TOP element and is repressed in growth-arrested cells (Hornstein et al. 1999a). Serum stimulation or HCMV infection activates mTORC1 and stimulates new PABP synthesis, which in turn promotes eIF4F assembly and 40S ribosome recruitment to the mRNA 5′ end (Perez et al. 2011; McKinney et al. 2012). The PABP repressor Paip2 regulates PABP1 function by (1) preventing its association with the mRNA poly(A) tail and (2) binding to PABP1 and preventing its association with eIF4G, both of which repress translation (Derry et al. 2006). Excess Paip2 that is not bound to PABP interacts with the E3 ubiquitin ligase EDD1, which targets Paip2 for proteolysis (Yoshida et al. 2006). While PABP depletion stimulates Paip2 degradation, how Paip2 responds to PABP accumulation is unknown and is depicted as a question mark. (B) Asynchronous NHDFs were mock-infected (0 hpi) or infected with HCMV at a multiplicity of 3. At the indicated times post-infection, total protein was isolated, fractionated by SDS-PAGE, and analyzed by immunoblotting with the indicated antisera. (C) As in B except at the indicated times post-infection, total RNA was isolated through Trizol extraction and subjected to RT-qPCR using the indicated primer sets. Each reaction product was normalized to the signal obtained using 18S rRNA, and the fold induction upon infection was calculated as described (Perez et al. 2011).