Figure 2.
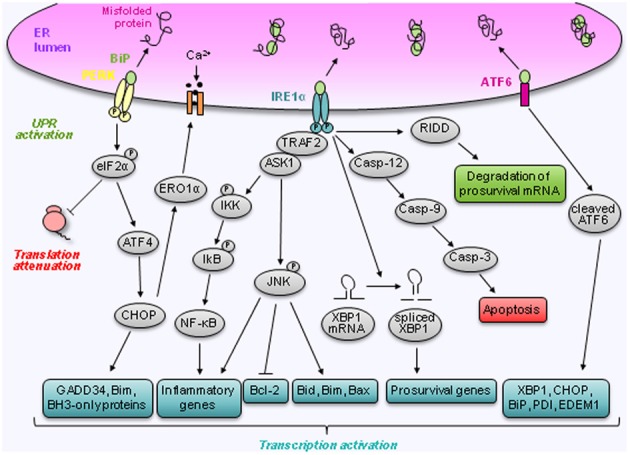
Regulation of prosurvival and apoptotic pathways by ER stress. Cells cope with ER stress by activating the unfolded protein response. This response is mediated via the dissociation of BiP from three ER transmembrane proteins IRE1α, PERK, and ATF6. Following dissociation of BiP, IRE1α becomes activated and induces splicing of XBP1 mRNA to XBP1s. IRE1α also activates JNK via TRAF2 and ASK1. Furthermore, activation of IRE1α has been linked to downstream NF-κB activation and RIDD, which can lead to the degradation of prosurvival mRNA. Finally, IRE1α controls the activation of the caspases signaling pathway. Like IRE1α, PERK becomes activated following BiP dissociation. Active PERK mediates its response via phosphorylation of eIF2α leading to a translational block and cap independent translation of ATF4. ATF4 induces CHOP which has multiple downstream targets that stimulate apoptosis and cell death. Following BiP dissociation, ATF6 is transported to the Golgi where it is cleaved into an active transcription factor. ATF6 regulates the expression of several genes involved in the unfolded protein response such as XBP1, CHOP, BiP, PDI, and EDEM1.
