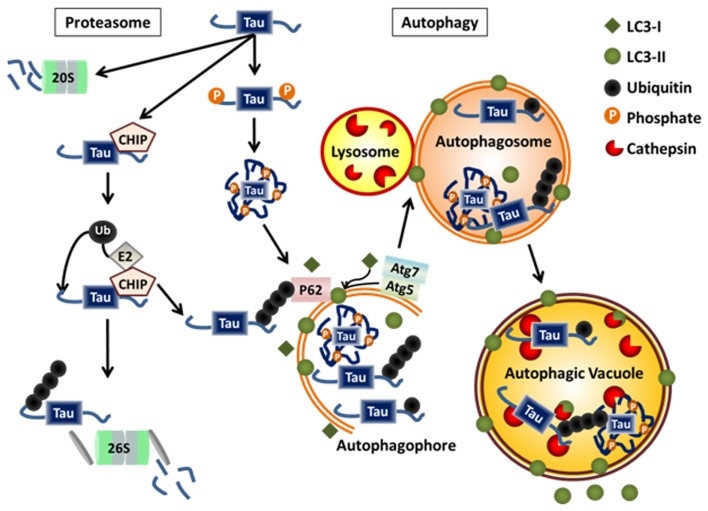
Figure 2.
Physiological degradation of tau. Tau is degraded by both the proteasome and autophagy systems. Targeting of tau to either system may be determined by the extent and nature of post-translational modifications, the folding state, the level of aggregation, and its interaction with chaperone proteins or ubiquitin ligases. Monomeric tau is natively unfolded making it a likely target for the 20S proteasome. Monomeric tau also interacts with the E3 ligase, CHIP, which can lead to its ubiquitylation and degradation via the 26S proteasome or autophagy. Certain cleavage products and phosphorylated forms of tau, as well as, monoubiquitylated tau and tau aggregates are selectively degraded by autophagy.