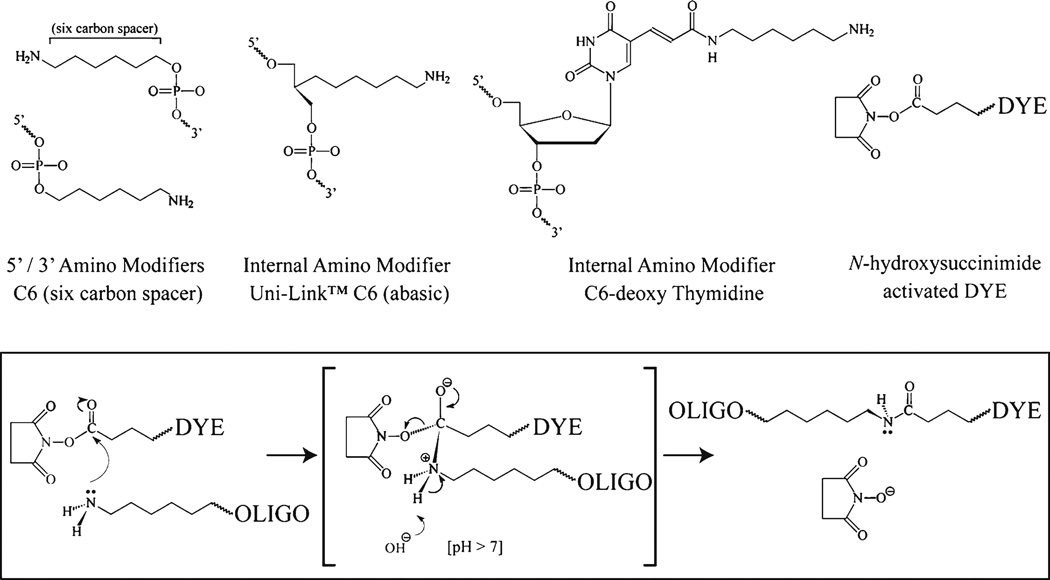
Fig. 5.
Labeling amine modified oligonucleotides using activated ester chemistry. A number of amino modifications are available for oligonucleotide synthesis; common amino modifiers are shown including end modifications (5′ and 3′) and internal modifications. The fluorophore (DYE) is purchased as an activated ester form to facilitate coupling to the oligonucleotide via the amine modification. The reactive chemistry is summarized; the amine reacts with the ester group resulting in the formation of a stable carboxamide bond between the oligonucleotide and the fluorescent dye. The reaction is facilitated by the enhanced stability of the N-hydroxysuccinimide leaving group.