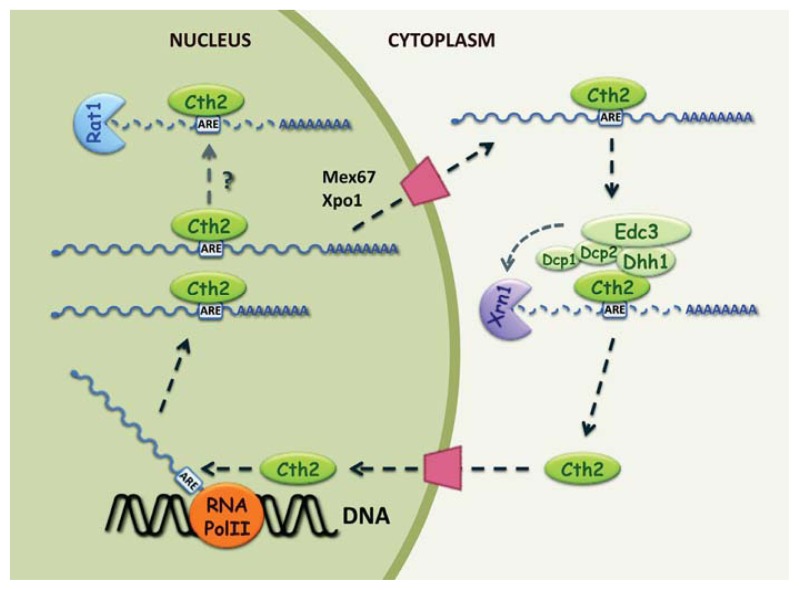
Figure 1.
Model for the post-transcriptional regulation of adenosine/uridine-rich element (ARE)-containing transcripts by Cth2 in response to Fe deficiency. This model proposes the following steps for the degradation of the SDH4 mRNA by Cth2 protein: (1) Docking and recognition: Cth2 co-transcriptionally binds to AREs in the nascent mRNA before polyadenylation occurs; (2) 3′ end processing: Cth2-binding to the AREs partially interferes with normal polyadenylation, leading to the synthesis of extended transcripts, which are preferentially degraded in a Cth2-dependent manner by a 5′ to 3′ exoribonuclease, either Rat1 in the nucleus or Xrn1 after transport to the cytoplasm; (3) Export: Cth2 bound to the transcript is translocated to the cytoplasm via mRNA export pathways that require Mex67 and Xpo1/Crm1; (4) Cytoplasmic degradation: Cth2 interacts with the RNA helicase Dhh1, which recruits the decapping enzymes and Xrn1 to the target mRNA that is degraded from 5′ to 3′; (5) Recycling and nuclear import: After mRNA degradation, Cth2 is released from the decay machinery and can potentially re-enter the nucleus to initiate a new cycle of ARE-mediated decay (AMD).