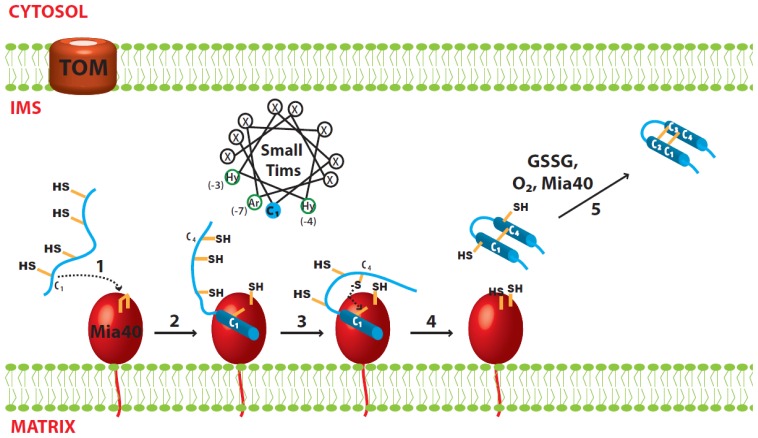
Figure 2.
Proposed model for oxidative folding of the small Tim proteins. (1) Nucleophilic attack of the C1 cysteine from the small Tim proteins; (2) Mia40-dependent folding initiated by hydrophobic interactions at the MISS/ITS segment, and stabilized by formation of an intermolecular disulfide bond; (3) Nucleophilic attack of the C4 cysteine from the small Tim proteins; (4) Mia40-independent folding. The second helix is formed using the first helix as scaffold, and stabilized by formation of the first intramolecular disulfide bond in the small Tim proteins (C1–C4); (5) The second disulfide bond could be formed by either oxygen, GSSG or Mia40.