Abstract
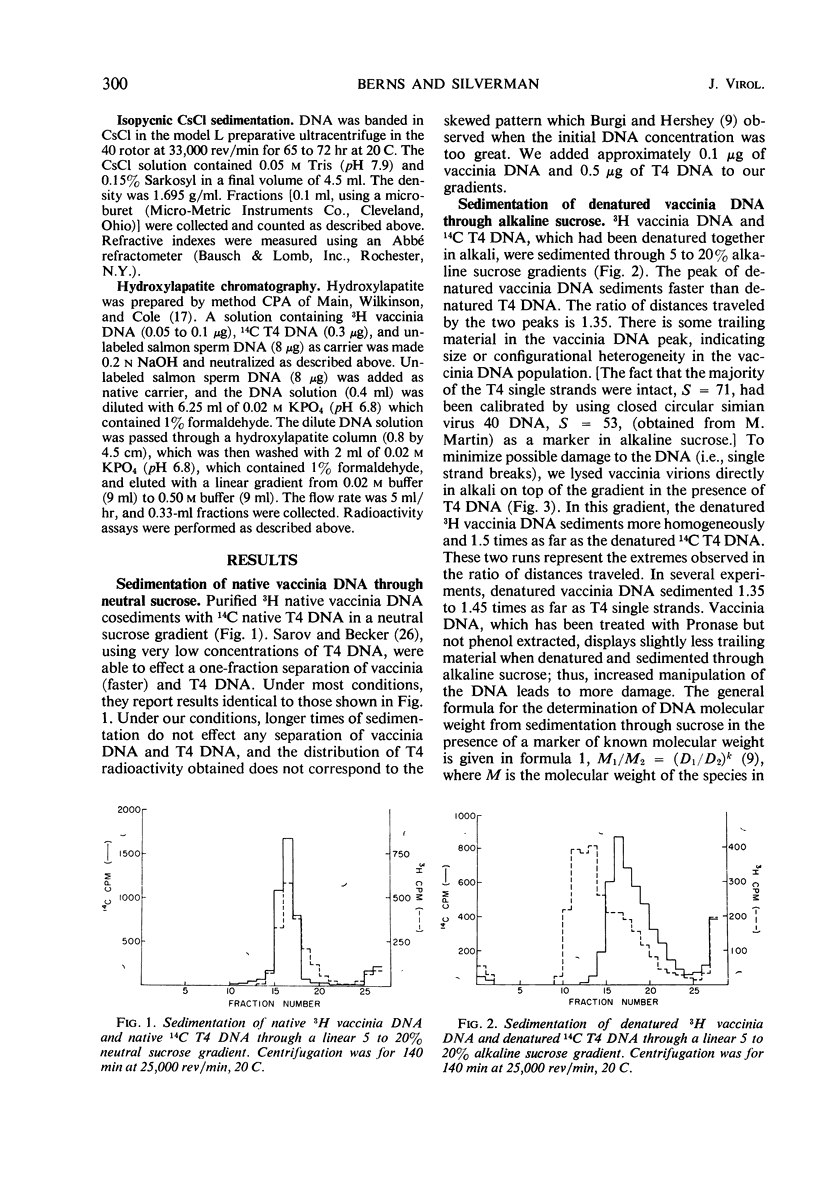
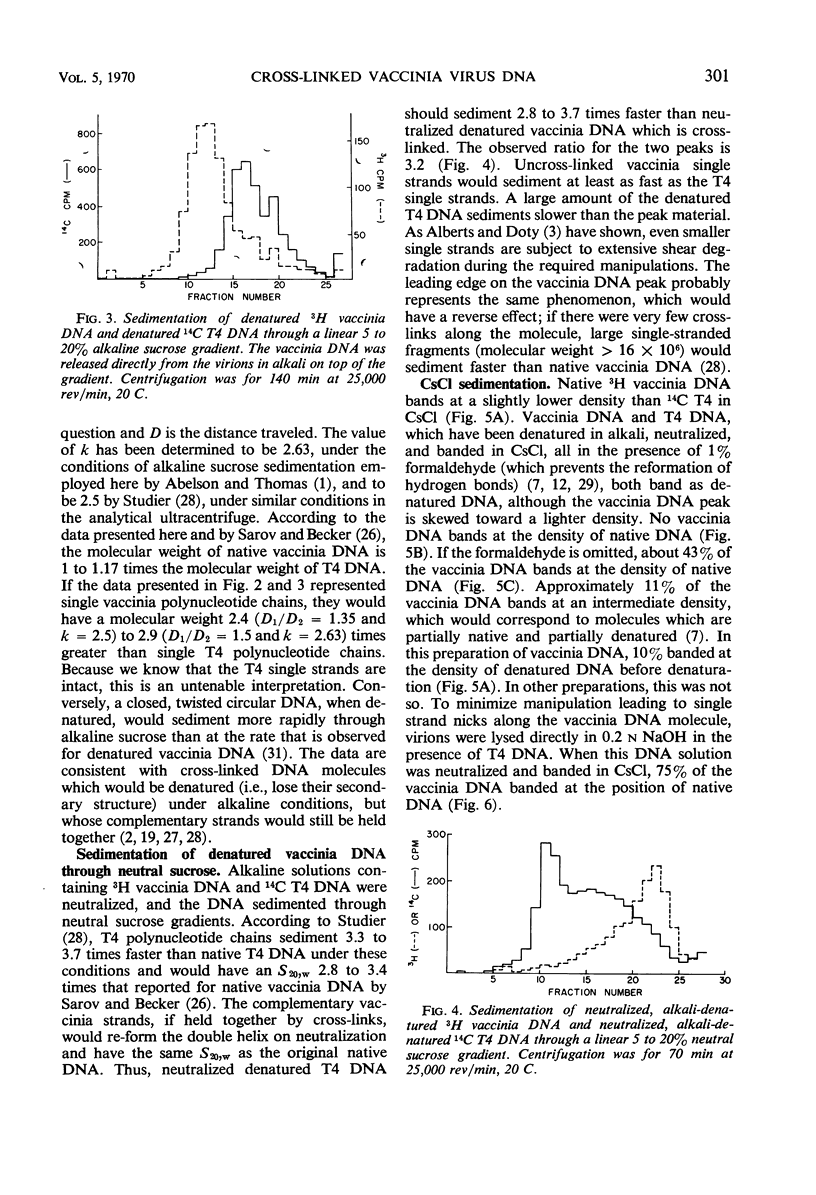
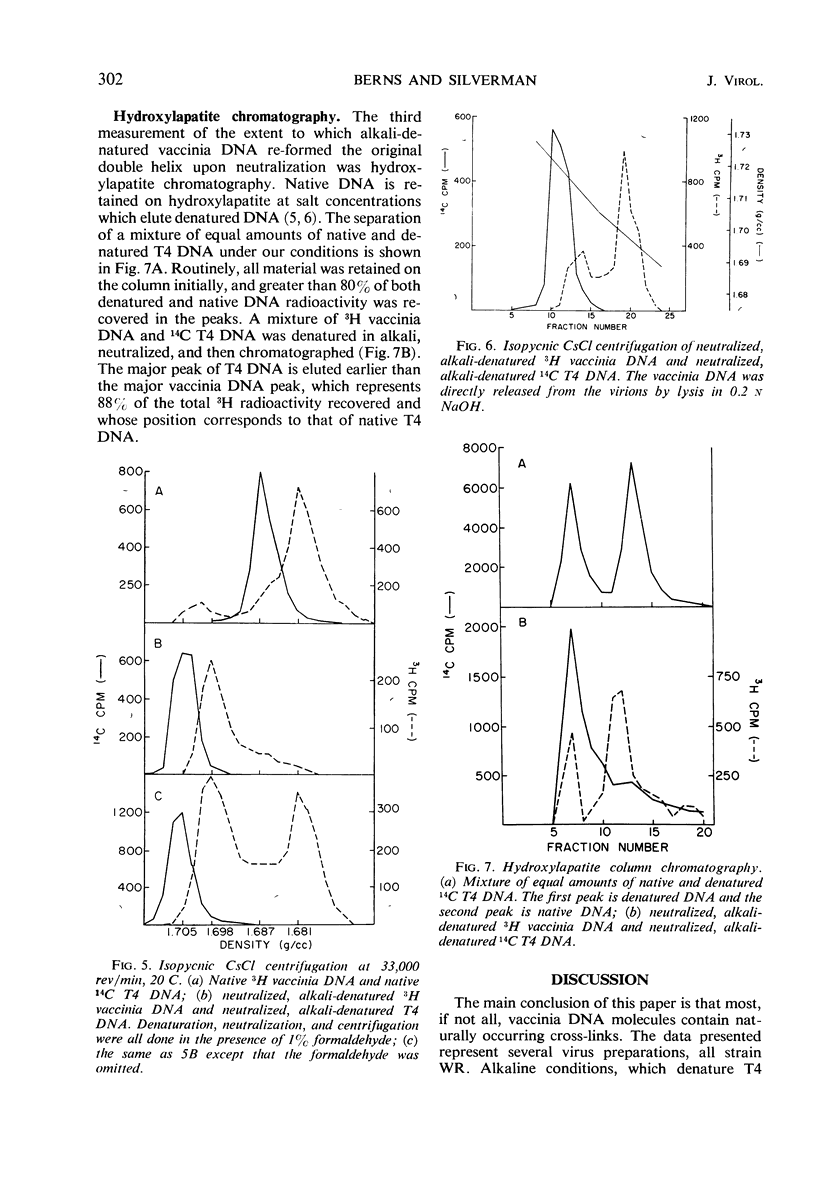
The molecular weight of native vaccinia deoxyribonucleic acid (DNA) is 1 to 1.17 times that of native T4 DNA. Sedimentation of denatured vaccinia DNA through alkaline sucrose gradients yields an apparent molecular weight greater than twice that of denatured T4 DNA, implying that the complementary strands of vaccinia DNA do not separate upon denaturation. When alkali-denatured vaccinia DNA is neutralized, it has the physical chemical properties of native DNA when tested by sedimentation through neutral sucrose gradients, banding in CsCl, and by hydroxylapatite chromatography. We conclude that almost all mature vaccinia DNA molecules contain a small number of naturally occurring cross-links.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., BURKE D. C. The nucleic acid contents of viruses. J Gen Microbiol. 1962 Feb;27:181–194. doi: 10.1099/00221287-27-2-181. [DOI] [PubMed] [Google Scholar]
- Alberts B. M. Characterization of a naturally occurring, cross-linked fraction of DNA. II. Origin of the cross-linkage. J Mol Biol. 1968 Mar 14;32(2):405–421. doi: 10.1016/0022-2836(68)90018-1. [DOI] [PubMed] [Google Scholar]
- Alberts B. M., Doty P. Characterization of a naturally occurring, cross-linked fraction of DNA. 1. Nature of the cross-linkage. J Mol Biol. 1968 Mar 14;32(2):379–403. doi: 10.1016/0022-2836(68)90017-x. [DOI] [PubMed] [Google Scholar]
- BERNARDI G. Chromatography of native deoxyribonucleic acid on calcium phosphate. Biochem Biophys Res Commun. 1961 Oct 23;6:54–57. doi: 10.1016/0006-291x(61)90184-x. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier M. R., Bernardi G. Residual transforming activity of denatured Haemophilus influenzae DNA. J Mol Biol. 1968 Mar 14;32(2):437–451. doi: 10.1016/0022-2836(68)90020-x. [DOI] [PubMed] [Google Scholar]
- Chevallier M. R., Bernardi G. Residual transforming activity of denatured Haemophilus influenzae DNA. J Mol Biol. 1968 Mar 14;32(2):437–451. doi: 10.1016/0022-2836(68)90020-x. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- GROSSMAN L., LEVINE S. S., ALLISON W. S. The reaction of formaldehyde with nucleotides and T2 bacteriophage DNA. J Mol Biol. 1961 Feb;3:47–60. doi: 10.1016/s0022-2836(61)80007-7. [DOI] [PubMed] [Google Scholar]
- HANAFUSA T., KAMAHORA J., HANAFUSA H. Transformation of ectromelia into vaccinia virus in tissue culture. Virology. 1959 Aug;8:525–527. doi: 10.1016/0042-6822(59)90053-4. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Jungwirth C., Dawid I. B. Vaccinia DNA: separation of viral from host cell DNA. Arch Gesamte Virusforsch. 1967;20(4):464–468. doi: 10.1007/BF01275228. [DOI] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- Moss B., Salzman N. P. Sequential protein synthesis following vaccinia virus infection. J Virol. 1968 Oct;2(10):1016–1027. doi: 10.1128/jvi.2.10.1016-1027.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagington J., Tee G. H., Smith J. S. Milker's nodule virus infections in Dorset and their similarity to orf. Nature. 1965 Oct 30;208(5009):505–507. doi: 10.1038/208505a0. [DOI] [PubMed] [Google Scholar]
- Nash H. A., Merril C. R. DNA of biotin-transducing lambda bacteriophage. J Mol Biol. 1969 Jul 28;43(2):357–359. doi: 10.1016/0022-2836(69)90277-0. [DOI] [PubMed] [Google Scholar]
- Rose J. A., Berns K. I., Hoggan M. D., Koczot F. J. Evidence for a single-stranded adenovirus-associated virus genome: formation of a DNA density hybrid on release of viral DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):863–869. doi: 10.1073/pnas.64.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALZMAN N. P., SHATKIN A. J., SEBRING E. D. Viral protein and DNA synthesis in vaccinia virus-infected HeLacell cultures. Virology. 1963 Apr;19:542–550. doi: 10.1016/0042-6822(63)90049-7. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sarov I., Becker Y. Studies on vaccinia virus DNA. Virology. 1967 Nov;33(3):369–375. doi: 10.1016/0042-6822(67)90112-2. [DOI] [PubMed] [Google Scholar]
- THOMAS C. A., Jr, BERNS K. I. The utility of formaldehyde in stabilizing polynucleotide chains from bacteriophage DNA. J Mol Biol. 1962 Apr;4:309–312. doi: 10.1016/s0022-2836(62)80008-4. [DOI] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]


