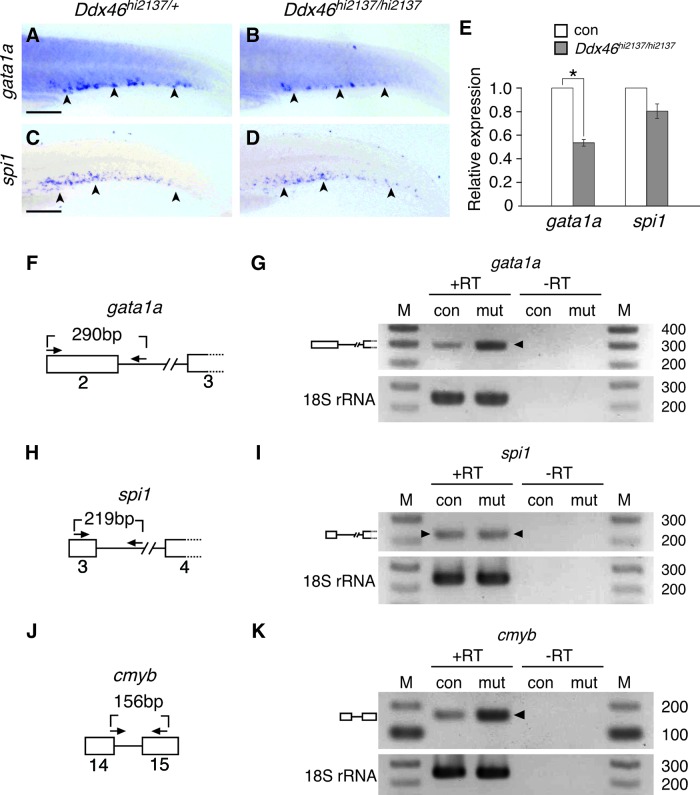
FIG. 8.
Expression and pre-mRNA splicing of gata1a, but not spi1, are defective in Ddx46hi2137/hi2137 mutants. (A–D) The expression of gata1a and spi1 was examined by whole-mount in situ hybridization at 3 dpf. All are lateral views, anterior to the left. The expression of gata1a in the CHT of Ddx46hi2137/hi2137 larvae (n=10/10) was markedly reduced compared with that of Ddx46hi2137/+ larvae (n=10/10) (arrowheads in A, B). In contrast, spi1 expression in the CHT of Ddx46hi2137/hi2137 larvae (n=10/10) was maintained compared with that of Ddx46hi2137/+ larvae (n=9/9) (arrowheads in C, D). Scale bars represent 100 μm. (E) Relative expression of gata1a and spi1 genes in control (con) larvae compared with that in Ddx46hi2137/hi2137 larvae at 3 dpf, by qPCR. Although no significant difference of spi1 expression was found between con and Ddx46hi2137/hi2137 larvae, gata1a expression in Ddx46hi2137/hi2137 larvae was significantly lower than that in con larvae. *P<0.01 by the Student's t-test. Error bars represent the standard error. (F–K) Schematic drawings of the gata1a, spi1, and cmyb pre-mRNA regions analyzed for splicing (boxes, exons; lines, introns; arrows, primers) (F, H, J). The splicing status of gata1a, spi1, or cmyb pre-mRNA was monitored by RT-PCR with the primers indicated in schemes (F), (H), or (J), respectively. The reverse primer for gata1a or spi1 mRNA was designed within the intron (F, H). The forward primer for cmyb crosses the exon14/intron14 boundary (J). Unspliced gata1a or cmyb mRNA was retained at a higher level in Ddx46hi2137/hi2137 mutant (mut) larvae than in con larvae (arrowhead in G=290 bp; arrowhead in K=156 bp). In contrast, the level of unspliced spi1 mRNA was indistinguishable between the mut larvae and con larvae (arrowheads in I=219 bp). Unspliced PCR products were verified by sequencing.+RT refers to the validation reaction itself, and −RT represents the respective control reaction without reverse transcriptase. 18S rRNA is a loading control. Control larvae were sibling WT or Ddx46hi2137/+ larvae, and they had normal phenotypes. qPCR, quantitative polymerase chain reaction; RT, reverse transcription. Color images available online at www.liebertpub.com/scd