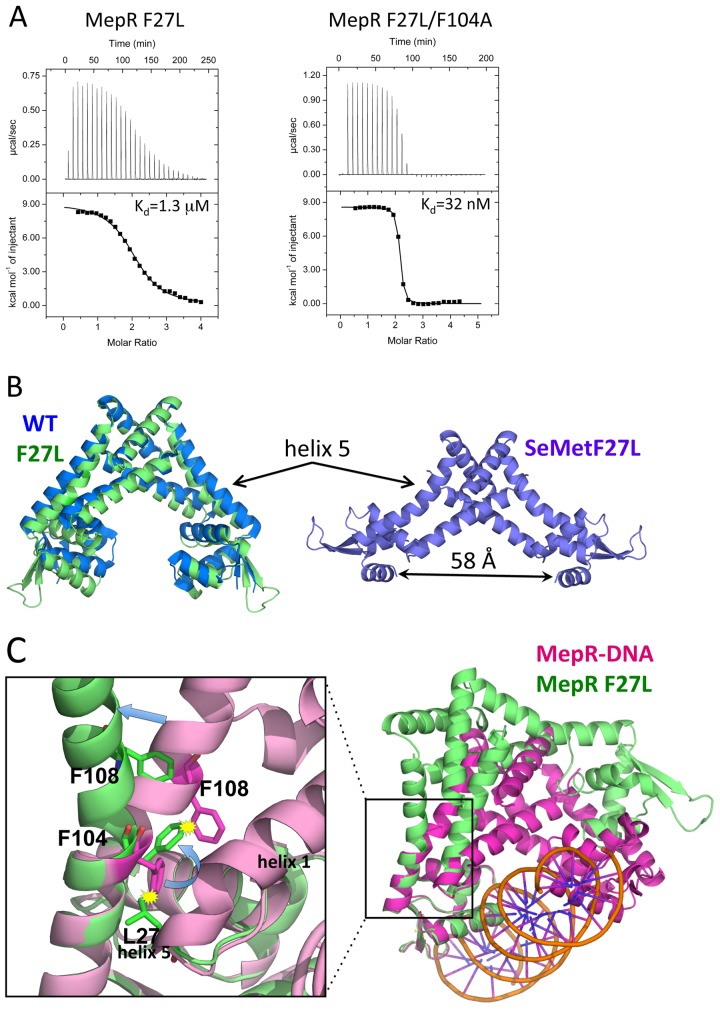
FIG 3 .
The effect of the F27L substitution on the linker region of MepR. (A) Titrations of mepR operator DNA with MepR(F27L) and MepR(F27L/F104A). The solid lines represent theoretical fits to the experimental data (closed squares); fitting parameters are provided in Table 1. (B) The structure of native MepR(F27L) superimposed on apoMepR (PDB accession code 3eco) and the structure of selenomethionine-substituted MepR(F27L). Helix 5, bent in both MepR(F27L) structures, is indicated by arrows; the distance between the recognition helices of the selenomethionine-substituted MepR(F27L) is 58 Å and indicated by a double-headed black arrow. (C) A structural alignment of the DNA binding domains of MepR-DNA model and native MepR(F27L). The area surrounding the mutation is magnified to demonstrate the details of the structural rearrangements in helix 5 caused by the F27L substitution.