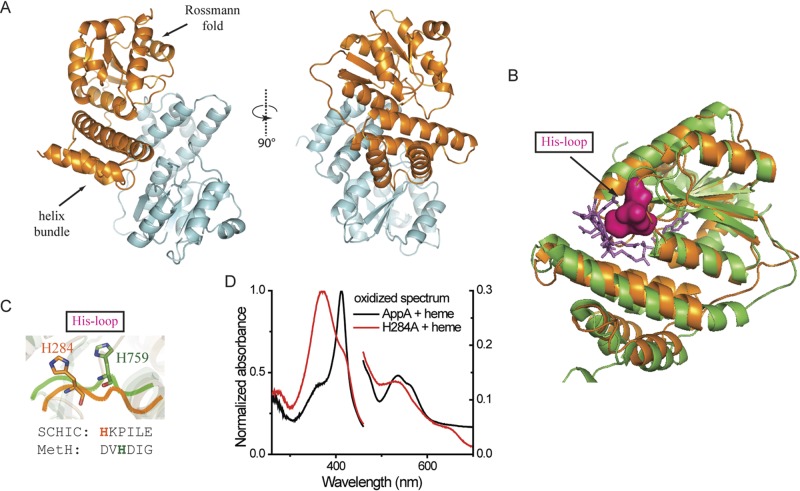
FIG 3 .
Crystal structure of SCHIC domain and His284 as the heme coordinating residue. (A) Crystal structure of SCHIC domain contains two subunits (subunit A shown in orange, and subunit shown B in light blue). (B) SCHIC domain (subunit A [orange]) shares similar overall conformation with E. coli MetH B12-binding domain (green). The His loop in MetH is highlighted in magenta, and the vitamin B12 molecule is shown as purple sticks. Subunit A of SCHIC domain is used for the structure comparison. (C) His284 in SCHIC (orange) and His759 in MetH (green) are located on the His loop region. Note the differences in their positions and orientations. (D) UV-visible spectrum of AppA-heme and AppAH284A-heme under aerobic conditions. The spectrum of AppA/AppAH284A is subtracted. Heme (5 µM) was incubated with 10 µM AppA/AppAH284A for at least 20 min before the spectrum was taken. Normalized absorbance is shown on both the right and left y axes.