FIG 4 .
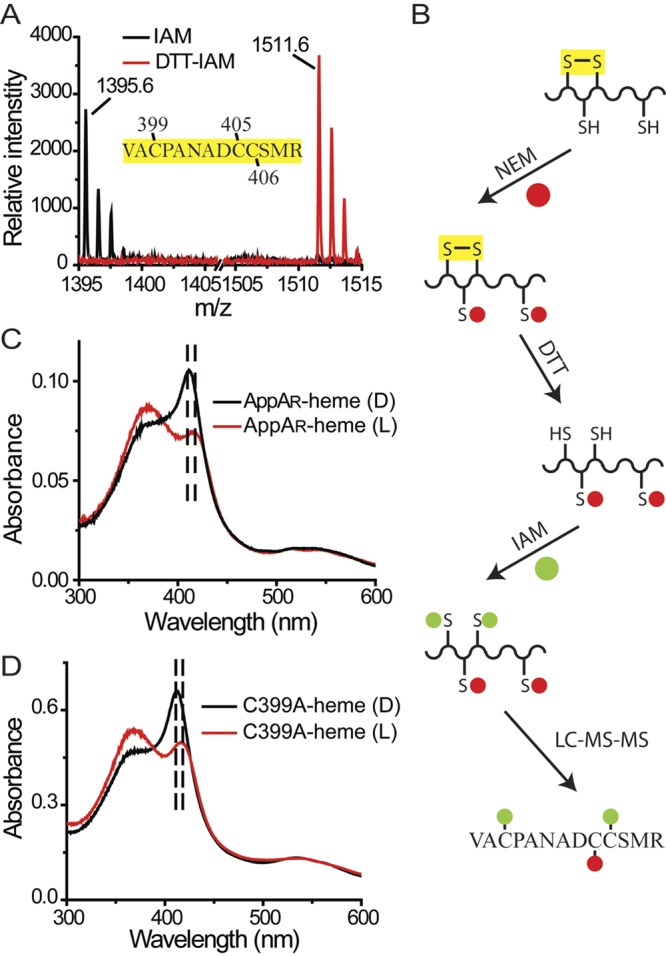
The disulfide bridge in the Cys-rich motif affects the SCHIC-heme interaction. (A) A disulfide bridge was identified within peptide AppA397-409. Under oxidized conditions, neither Cys399 nor Cys406 can be modified by iodoacetamide (IAM). Under reduced conditions, the disulfide bridge formed between Cys399 and Cys406 is reduced, and both residues can be modified by iodoacetamide (DTT-IAM). (B) LC-MS-MS with double-labeled AppA identified the Cys399-Cys406 disulfide bond (see Fig. S2 in the supplemental material). (C) The cysteine residues in AppA are reduced by DTT (AppAR). When light excited, the Soret peak of AppAR-heme is redshifted from 412 nm to 418 nm. (D) When light excited, the Soret peak of AppAC399A-heme is redshifted from 412 nm to 418 nm.
