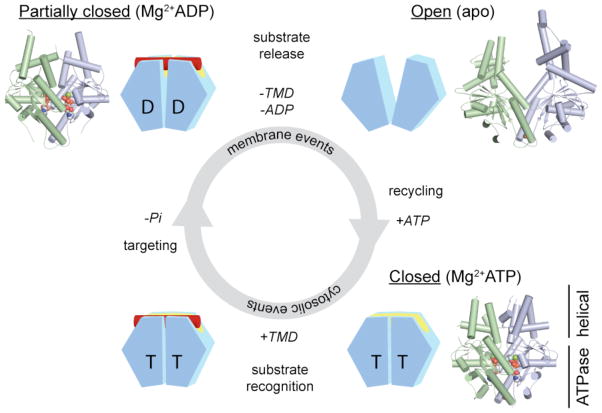
Figure 2. Nucleotide-dependent conformational changes in the Get3 homodimer.
Each Get3 monomer comprises two distinct regions: an ATPase- and an α-helical subdomain. In the presence of ATP, the Get3 helical subdomains become intimately associated, forming an extended composite hydrophobic groove (see FIG. 3) that recognizes and binds to the TMD of a TA substrate (red). ATP hydrolysis, which occurs at some stage prior to release of the TA substrate at the ER membrane, produces an ADP-bound Get3 homodimer that is partially closed. Following release of the TA substrate and ADP, Get3 shifts back to an open conformation. Subsequently, ATP binding allows recycling of Get3 back to the cytosol in a closed conformation. The insets show crystal structures of the fungal Get3 homodimer in the nucleotide-free (PDB ID 2WOO), Mg2+ADP-bound (PDB ID 3IQX) and Mg2+ADP•AlF4−-bound (PDB ID 2WOJ) states.