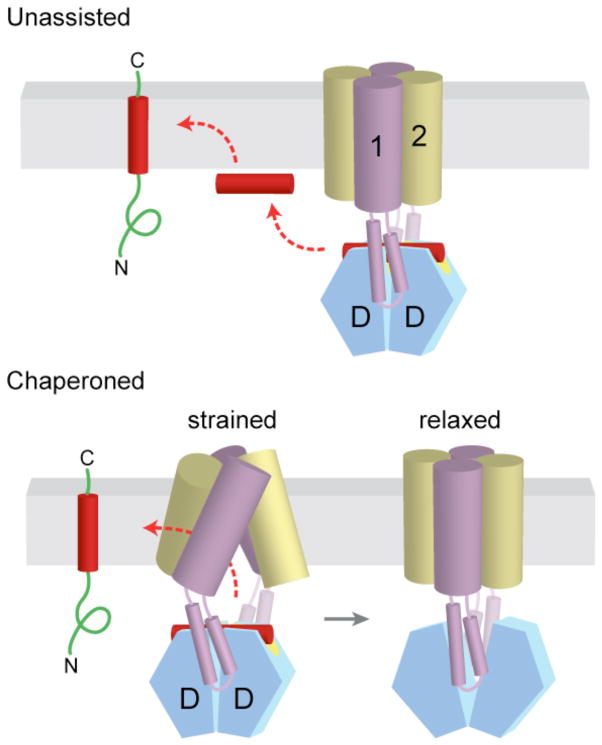
Figure 6. Alternative models for insertion of TA proteins into the ER membrane.
a | After release from Get3, the TA substrate might transiently associate with the membrane surface before inserting spontaneously into the lipid bilayer. b | Alternatively, the transmembrane domains (TMDs) of Get1 and Get2 may interact directly with the TA substrate to ‘chaperone’ it into the bilayer. In the model shown here, binding of two Get1 subunits to the partially closed targeting complex results in a ‘strained’ configuration of the Get1–Get2 receptor complex. This arrangement might provide a hydrophobic gate through which the TMD could diffuse into the bilayer. ‘Wedging open’ the Get3 dimer for substrate release would then allow the Get1–Get2 receptor complex to ‘relax’ back into a low-energy configuration that is sealed off from the cytosol. Thus, conformational changes in Get3 might be coupled to those of the receptor TMDs to promote TA substrate integration.