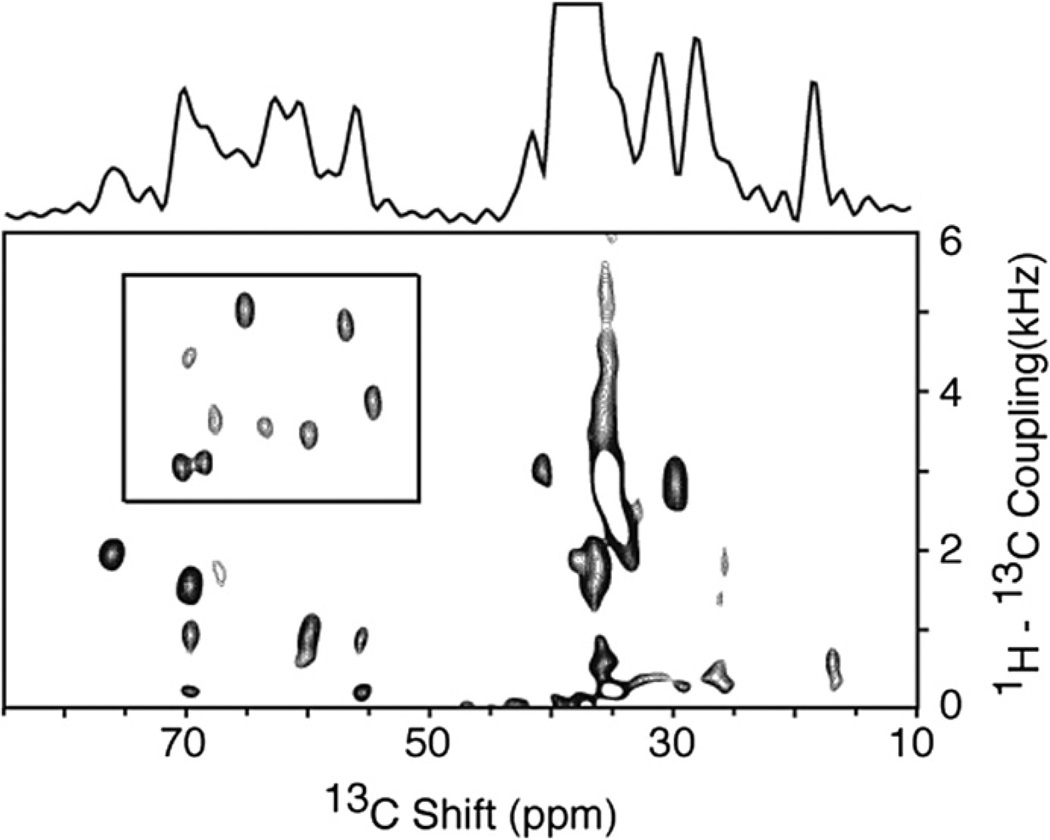
Fig. 4.
Two-dimensional 1H–13 C PISEMA spectrum of the membrane-bound form of the 46-residue Pf1 coat protein in magnetically aligned phospholipid bilayers where the protein is labeled with 13Cα and N at all valine, leucine and isoleucine residues (13Cα, N VLI Pf1). The spectrum was obtained using the pulse sequence in Fig. 1a. All of the signals in the box are from 1H–13Cα dipolar couplings. Nearly all of the other intensity observed in the spectrum in from the natural abundance signals from the phospholipids in the sample. Ninety six scans were acquired for each of 128 points in the indirect dimension. The recycle delay was 5 s and the temperature 40 °C. The two-dimensional data set was zero filled to 1K data points and multiplied by a sine bell window function in both dimensions before double Fourier transformation.