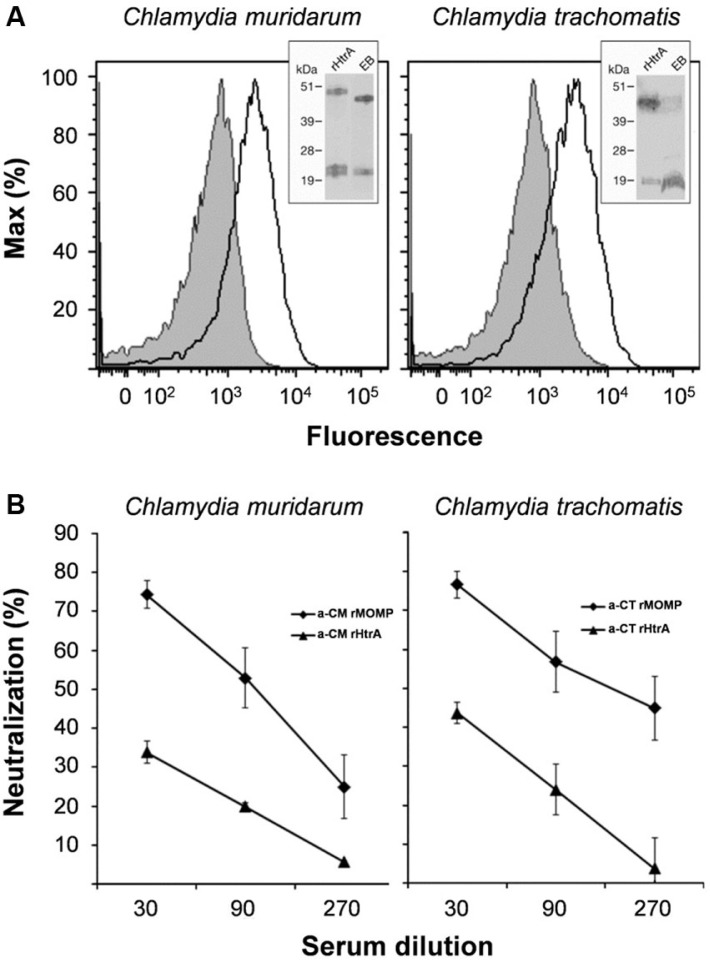
Fig. 1.
Surface-exposed Chlamydia HtrA does not elicit neutralizing antibodies. (A) HtrA Chlamydia HtrA is expressed on the EB surface. Purified CM (left panel) and CT (right panel) EBs were incubated with sera raised against CM rHtrA or CT rHtrA (empty peaks) or anti-E. coli contaminants (shaded peaks) and the antibody binding on EB surface was detected by flow cytometry using a R-Phycoerythrin-conjugated secondary antibody. Inset panels: Western blot analysis of HtrA expressed by EBs. Total extracts from CM or CT EBs (approximately 107) and purified CM or CT rHtrA were separated on SDS-PAGE, transferred to nitrocellulose membranes and incubated with the corresponding rHtrA antiserum. Molecular weight standards are reported on the left side of each panel. (B) Antibodies elicited by CM rHtrA do not neutralize Chlamydia infectivity in vitro. Anti-CM rHtrA and anti-CT rHtrA antibodies were pre-incubated at different dilutions with infectious CM EBs (left panel) and CT EBs (right panel), respectively, and used to infect LLC-MK2 cell monolayers, as compared with EBs treated with corresponding dilutions of pre-immune sera. As positive controls, anti-CM rMOMP and anti-CT rMOMP were also used simultaneously. The antiserum neutralization activity was determined by measuring the reduction of the number of inclusions generated by antibody-opsonized EBs after correction for background inhibition observed with pre-immune sera. The graphs represent the percent neutralization averaged from triplicate experiments, with standard deviations.