Figure 4.
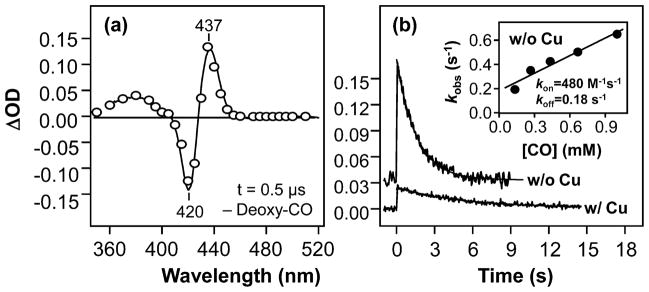
Transient absorption difference spectrum (t = 0.5 μs) (a) and the kinetic traces (b) obtained following photodissociation of CO from CuBMb. The protein and CO concentrations were 5.2 μM and 1 mM, respectively. The spectrum in (a) was obtained in the absence of Cu+; the solid spectrum represents the difference spectrum calculated from the equilibrium deoxy and CO-bound spectra shown in Figure 2a. The kinetic traces in (b) were obtained in the presence and in the absence of 64 μM Cu+, as indicated; the solid curves are the single-exponential fits of the data. In the presence of Cu, the observed rate constant for CO rebinding is 0.19 s−1. The inset in (b) shows the plot of the observed rate constant of the Cu+-free CuBMb as a function of CO concentration; the kon and koff values were determined from the slope and intercept of the best-fit line (solid line) to be 4.8 × 102 M−1 s−1 and 0.18 s−1, respectively.
