Abstract
Human-induced pluripotent stem cells (hiPSCs) can differentiate into functional cardiomyocytes (iCell Cardiomyocytes) with ion channel activities that are remarkably similar to adult cardiomyocytes. Here, we extend this characterization to cardiac ion transporters. Additionally, we document facile molecular biological manipulation of iCell Cardiomyocytes to overexpress and knockdown transporters and regulatory proteins. Na/Ca exchange (NCX1) and Na/K pump currents were recorded via patch clamp, and Na/H and Cl/OH exchanges were recorded via oscillating proton-selective microelectrodes during patch clamp. Flux densities of all transport systems are similar to those of nonrodent adult cardiomyocytes. NCX1 protein and NCX1 currents decline after NCX1 small interfering (si)RNA transfection with similar time courses (τ ≈ 2 days), and an NCX1-Halo fusion protein is internalized after its extracellular labeling by AlexaFluor488 Ligand with a similar time course. Loss of the cardiac regulatory protein phospholemman (PLM) occurs over a longer time course (τ ≈ 60 h) after PLM small interfering RNA transfection. Similar to multiple previous reports for adult cardiomyocytes, Na/K pump currents in iCell Cardiomyocytes are not enhanced by activating cAMP production with either maximal or submaximal cytoplasmic Na and using either forskolin or isoproterenol to activate adenylate cyclases. Finally, we describe Ca influx-dependent changes of iCell Cardiomyocyte capacitance (Cm). Large increases of Cm occur during Ca influx via NCX1, thereby documenting large internal membrane reserves that can fuse to the sarcolemma, and subsequent declines of Cm document active endocytic processes. Together, these results document a great potential of iCell Cardiomyocytes for both short- and long-term studies of cardiac ion transporters and their regulation.
Keywords: human-induced pluripotent stem cells, iCell Cardiomyocytes, ion transporters, sodium-calcium exchange, sodium-potassium pumps, phospholemman
embryonic cardiac myocyte cultures have been used effectively since the 1970s for long-term studies of cardiac function and protein turnover (e.g., Refs. 22, 34, 35). Adult cardiac myocyte cultures have been used since the early 1980s with a still greater range of experimental goals (e.g., Refs. 27, 28, 32). In spite of impressive advances with a variety of cardiac myocyte cultures (17), the utility of cultured myocytes, especially adult cultures (31), has remained limited for a variety of reasons (12). In contrast to intact cardiac tissue, isolated adult cardiac myocytes are in general fragile and usually do not support sustained contractile activity. As with most differentiated cell types, extensive structural and functional changes occur during culture of adult cardiomyocytes (32). Furthermore, transfection and genetic manipulation of cultured adult cardiomyocytes are difficult. Common alternatives, such as neonatal cardiac myocyte cultures (3) and immortalized cardiomyocyte cell lines (39), allow more sustained and experimentally reliable studies. However, they also bring more equivocal relevance to physiology of the adult human heart. In this light, recent progress in generating stem cell-derived cardiomyocytes raises entirely new opportunities for basic as well as therapy-driven research (33, 42).
The minimum list of desirable cardiomyocyte culture traits for most biochemical and functional studies is extensive and challenging. For biochemical purposes, the culture must be homogeneous. To be relevant to the intact heart, the culture should form a syncytium. Further, it should express proteins that are characteristic of adult cardiac myocytes, it should be functionally stable, it should be senescent but nevertheless metabolically active, and it should allow molecular biological manipulations to overexpress and knockdown specific proteins. For studies of cardiac ion channels and transporters, cardiomyocytes must allow convenient application of patch-clamp techniques. Finally, to be directly relevant to human physiology and disease, the ideal culture will be of human origin. In this light, cardiomyocytes derived from human-induced pluripotent stem cells (hiPSCs) by a number of different approaches (11, 13, 18, 38) are increasingly attractive models.
Among the different approaches to generating hiPSC-derived myoyctes, one of the most promising uses an hiPSC line engineered to ultimately permit high volume selection for cardiomyocytes by expressing blasticidin resistance under the control of the cardiac myosin heavy chain 6 (MYH6) promoter (25). Cells derived in this way show remarkably human-like electrophysiology (25, 30). Membrane currents that have been characterized include sodium, L-type calcium, hyperpolarization-activated, transient outward potassium, inward rectifier potassium, and the rapidly and slowly activating components of delayed rectifier potassium currents (25). The potential utility of similar cultures in automated imaging platforms to characterize hypertrophic and toxicity profiles is documented (7), and the maturation of similar cultures to a nearly adult myocyte-like phenotype over several months has recently been described (16). In the present article we extend the use of hiPSC-derived myoyctes to cardiac ion transporters, including Na/K pump, Na/Ca exchange currents, Na/H exchange (NCX1), and Cl/OH exchange. In addition, we demonstrate overexpression and quantitative knockdown of selected cardiac proteins by standard molecular biological methods. The results demonstrate that this cardiomyocyte culture system has many features required for substantively improved studies of cardiac ion transporters, in particular for studies of regulatory proteins and long-term regulatory pathways that impact the expression and function of membrane transporters.
METHODS
Generation of iCell Cardiomyocytes.
Functional cardiomyocytes (iCell Cardiomyocytes) were obtained from Cellular Dynamics International (Madison, WI). Generation of the hiPSC cell line was described previously in detail (25). In brief, human fibroblast cell lines were reprogrammed by retroviral expression of sox7, oct4, nanog, and lin28. hiPSC clones were then generated to exhibit blasticidin resistance under control of myosin heavy chain 6 (MYH6). A neomycin-resistance gene driven by the constitutive phosphoglycerate kinase promoter was included in the targeting vector to allow selection of genomic integrants by inclusion of neomycin in the iPSC culture medium. Neomycin-resistant iPSC colonies were subjected to genomic DNA PCR screening to identify correctly targeted homologous recombinants. hiPSC aggregates were formed from single cells and cultured in suspension. Upon observation of beating cardiac aggregates, cultures were subjected to blasticidin selection at 25 μg/ml to enrich the cardiomyocyte population. Cardiomyocyte aggregate cultures were maintained in DMEM with 10% FBS during cardiomyocyte selection through the duration of the culture before cryopreservation at 30–32 days of culture.
Cardiomyocyte cultures.
Neonatal rat cardiomyocytes were prepared essentially by an established protocol (3), and the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center approved the protocols employed and the experiments. The hiPSC cells (iCell Cardiomyocytes; Cellular Dynamics International) were plated to a density of 150,000–300,000/well on 0.1% gelatin-coated 12-well cell culture dishes using iCell Cardiomyocyte Plating Medium (iCPM; Cellular Dynamics International), essentially as described previously (25). For imaging and immunofluorescence studies, cells were cultured equivalently on gelatin coated coverslips. Syncitial monolayers of beating cells formed within 48 h. The iCPM medium was then removed and replaced with Maintenance Medium (iCMM; Cellular Dynamics International). Cells were then further incubated at 37°C at 7% CO2 and maintained for up to 4 wk with medium changes every 48–72 h. Transfections of DNA and small interfering (si)RNA of hiPSC cells were performed using TransIT-LT1 and siQUEST (Mirus), respectively. In the experiments using DNA transfection to express fluorescent proteins, the transfection efficiency was 15–25%. In small interfering (si)RNA knockdown experiments, siGLO Green Indicator (D-001630-01-05; Dharmacon), protein loss evaluated by Western blotting, and loss of NCX1 transport activity, all indicated that transfection was >85% efficient. Therefore, it was not necessary to use optical methods to select cells for electrophysiological measurements in those experiments. Before patch-clamp experiments, cells were removed from dishes by trypsin treatment (0.25%) and maintained thereafter in suspension at room temperature in serum-containing medium. All experiments were performed within 3 h after deplating.
Electrophysiological methods, solutions, and sphingomyelinase.
Patch-clamp and ion selective electrode methods were all described previously (8, 20, 40, 41), and all recordings were performed at a holding potential of 0 mV. In brief, the solutions employed for NCX1 and Na/K pump current measurements minimized all other currents. The extracellular solution contained the following (in mM): 115 N-methyl-d-glucamine (NMG), 4 MgCl2 or 2 MgCl2 + 3 CaCl2, 15 TEA-OH, 10 HEPES, and 0.5 EGTA, set to pH 7.0 with aspartate. The cytoplasmic solution contained the following (in mM): 80 NMG, 20 TEAOH, 15 HEPES, 40 NaOH, 0.5 MgCl2, 0.5 EGTA, 0.25 CaCl2, 8 MgATP, 2 TrisATP, and 0.2 GTP, set to pH 7.0 with aspartate. Free Ca of cytoplasmic solutions was calculated to be 0.5 μM, and free Mg was calculated to be 0.4 mM using WEBMAX EXTENDED (http://www.stanford.edu/∼cpatton/webmaxc/webmaxcE.htm). The extracellular solution for Na/H exchange measurements contained the following (in mM): 140 NaCl, 2 CaCl2, 2 MgCl2, and 0.1 Tris, set to pH 8.2, and the pipette solution contained the following (in mM): 150 KOH, 50 PIPES, 59 aspartate, 1 EGTA, 0.5 MgCl2, 8 MgATP, and 2 TrisATP, set to pH 6.8. The extracellular solution for Cl/OH exchange measurements contained the following (in mM): 140 KCl or K-sulphamate, 2 CaCl2, 2 MgCl2, and 0.1 2-(N-morpholino)ethanesulfonic acid, set to pH 6.5, and the pipette solution contained the following (in mM): 140 KOH, 50 HEPES, 59 aspartate, 1 EGTA, 0.5 MgCl2, 8 MgATP, and 2 TrisATP, set to pH 7.6 with sulfamate. All chemicals were of the highest grade available from Sigma-Aldrich. Purified Bacillus cereus sphingomyelinase (SMase) was a gift of J. Sakurai (Tokushima Bunri University and University of Tokushima, Tokushima, Japan).
Transfection and constructs.
Transfections of hiPSC cells were performed using TransIT-LT1 and siQUEST (Mirus), as noted above, following manufacturer protocols. The extracellular NCX1.1-HaloTag (Promega) construct was a generous gift of Debora Nicoll (University of California, Los Angeles) utilizing the same external glycosylation site as a pHluorin construct developed by us (20). The construct to generate an extracellular fusion of the pH sensitive fluorophore pHluorin with PLM was developed similarly to the NCX1-pHluorin fusion (20). Briefly, using site-directed mutagenesis (Stratagene), we inserted a Cla1 cleavage site just before the NH2-terminal glutamate and after the signal sequence in the canine PLM construct (gift of J. Y. Cheung, Jefferson Medical College). We then inserted the pHluorin construct as described previously (20). siRNA constructs were purchased from Dharmacon as follows: NCX, siGENOME Human SLC8A1 (M-007620-01-0005); PLM, siGENOME Human FXYD1 (M-011977-00-0005); Mock transfection, siGENOME Non-Targeting siRNA pool#2 (D-001206-14-05); and siGLO Green Indicator (D-001630-01-05) to evaluate transfection efficiencies. For electrophysiological recordings, cells were removed at selected times with Cellgro 0.25% Trypsin-EDTA (Corning). For Western blotting, cells were lysed in RIPA as described below.
Immunofluoresence imaging.
For immunofluorescence, iCells were plated into 12-well dishes containing 0.1% gelatin-coated coverslips at a density of 200,000 cardiomyocytes per well. At room temperature, cells were washed once with PBS and fixed with 4% paraformaldehyde for 15 min, rinsed two times with PBS for 5 min, and permeabilized with 0.1% Triton X-100 in PBS for 10 min. Cells were blocked for 15 min at 37°C with TBS containing 0.1% Tween and 10% FBS. Coverslips were transferred to a sealed moist box, placed on parafilm, and incubated in blocking buffer with mouse anti-sarcomeric α-actinin primary antibody (EA-53; Sigma; 1:100) overnight at 4°C. Coverslips were transferred back to the dish and washed three times with TBS-Tween for 15 min. Goat-anti-mouse secondary with Cy5 (H+L) (Jackson ImmunoResearch, 1:400) was applied in blocking buffer for 2 h at room temperature. The iCells were washed once with TBS-T, and mounted on glass slides using Vectashield mounting media with DAPI (H-1200, Vector Laboratories). Imaging was performed using a ×63 HCX Plan Apochromat, NA 1.45, glycerin lens on a Resonance Scanning Confocal Microscope (SP5; Leica). Imaging data were processed and quantified using ImageJ (National Institutes of Health).
Live-imaging and life-time analysis.
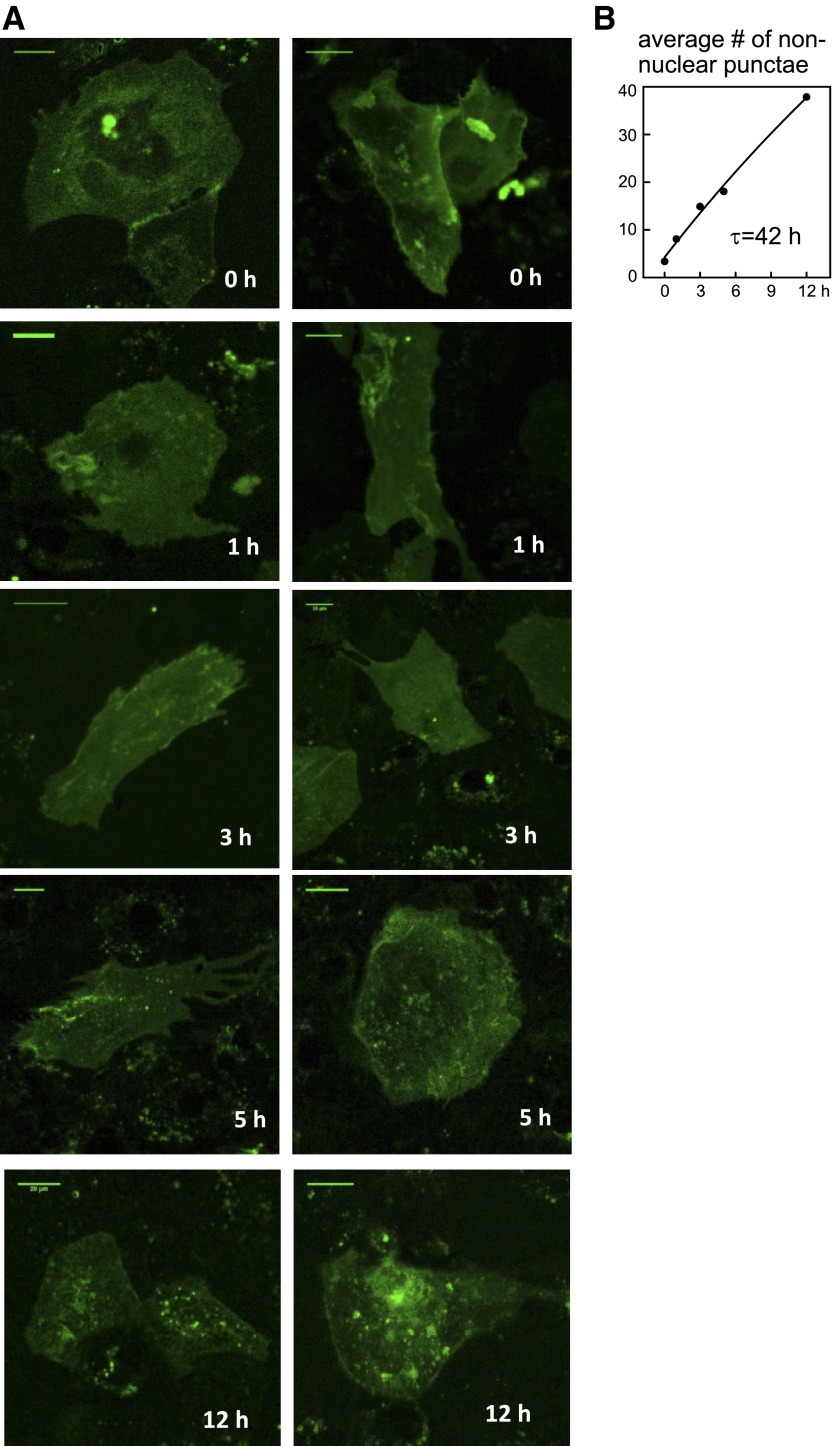
For live-imaging, iCell Cardiomyocytes were grown on gelatin-coated 12-well glass bottom culture dishes (MatTek). Cells were transfected with PLM-pHluorin or NCX1.1-HaloTag. After 4 days, confocal imaging was performed using a Nikon TE2000-U with a ×60 oil immersion, 1.45-NA objective. A 40-mW 163-CO2 laser (Spectra Physics; Newport) operating at 488 nm and 3% of maximum capacity was used for excitation. Resolution was set to 512 × 512, yielding <2-s exposure times with a pinhole of 150 μm. Cells were imaged in PBS solution buffered to a pH of 7.4 or 6.2, as indicated. For lifetime pulse-chase analysis of NCX1.1-HaloTag, cells were labeled with 1 μM of the impermeable HaloTag AlexaFluor 488 Ligand (G1002; Promega) for 15 min in culture media at 37°C. The cells were washed several times in media, and imaging was performed at selected time points, as indicated. Backgrounds were subtracted in ImageJ, and nonnuclear punctae were quantified within a binary image defined as any particle between 0.5 and 5 μm2.
Western blotting.
Cells were lysed with cold RIPA buffer (150 mM NaCl, 1% NP40, 0.5% deoxycholate, and 0.1% SDS, 50 mM Tris·HCl) containing protease inhibitor cocktail (Roche). Protein concentrations were determined by MicroBCA Protein Assay Kit (Thermo). Samples for NCX1 detection were prepared with nonreducing SDS sample buffer and incubated at 37°C for 30 min before running on a 7% SDS-PAGE gel. For PLM, reducing buffer was used and heated to 92°C for 5 min. Samples were run on a 12% SDS-PAGE gel. All proteins were then transferred onto nitrocellulose membrane overnight at 4°C. For immunodetection, proteins were probed with monoclonal R3F1 (NCX1; 1:200) and/or anti-actin (Chemicon; 1: 2,000) and polyclonal C2-PLM (1:10,000; a gift of J. Y. Cheung, Temple University). Peroxidase-conjugated anti-mouse IgG (Bio-Rad) and anti-rabbit IgG (GE Healthcare) was used as the secondary antibody at 1:3,000. Peroxidase-labeled proteins were visualized via chemiluminescence (ECL+; GE Healthcare).
RESULTS
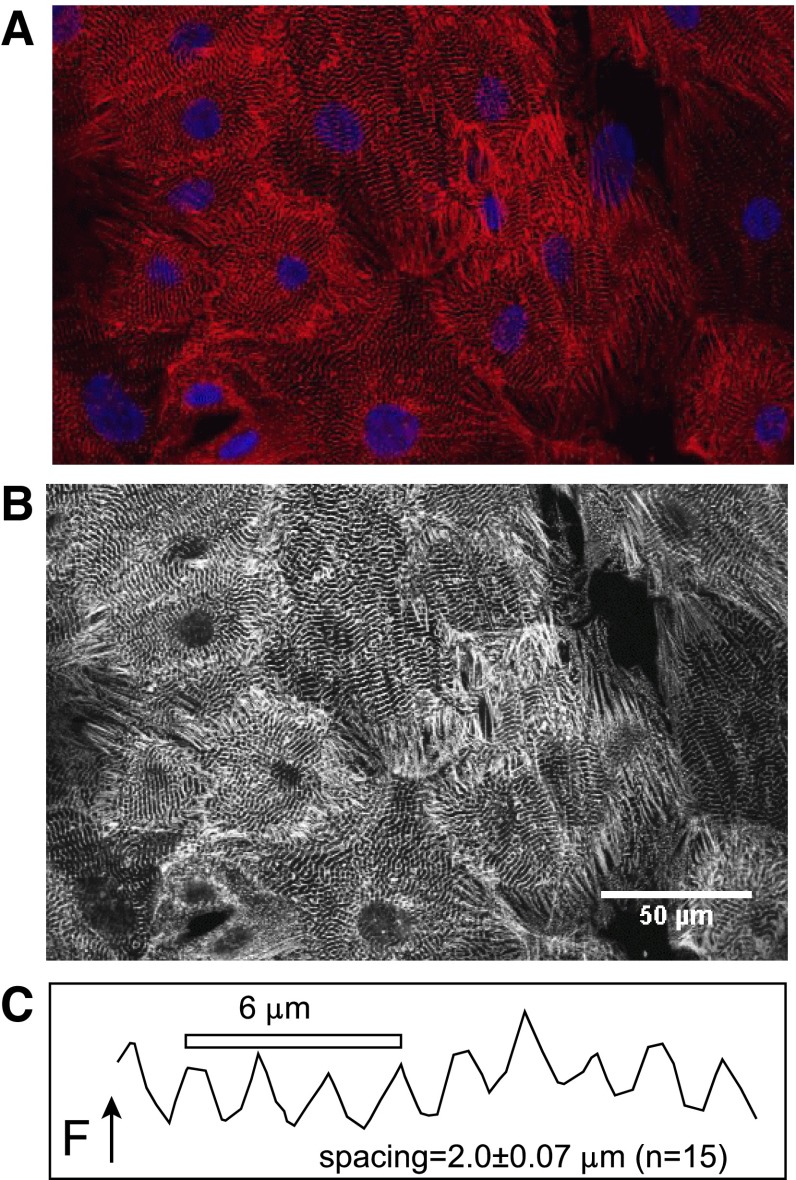
Figure 1 demonstrates the filament structure of iCell Cardiomyocytes revealed by staining cells with TRITC-labeled anti-α-actinin (red in A; white in B) simultaneous with DAPI (blue in A) to localize nuclei. Actinin is clearly organized in periodic bands. From 15 measurements of segments containing 8–10 bands, the average band spacing was 2.0 ± 0.07 microns (Fig. 1C), as expected for a developed muscle phenotype. The great majority of cells are mononuclear. While these images are suggestive of a rather homogenous cell culture, the isolation of detached single cells by trypsin treatment reveals that cardiomyocytes have a substantially wide range of dimensions and morphologies. Furthermore, functional analysis of ion transporter function reveals variability that in general correlates with cell morphology.
Fig. 1.
iCell Cardiomyocytes stained with EA-53 anti-sarcomeric α-actinin antibody and mouse monoclonal secondary-CY5 together with DAPI nuclear staining. A: dual fluorescence image of iCell Cardiomyocyte culture illustrating myofilaments and mononucleation of cells. B: high contrast black and white depiction of the same image to illustrate myofilament arrangements. C: typical α-actinin spacing from the black and white image determined from fluorescence, F, profiles along lines passing perpendicularly through striations. From 15 similar measurements, the average spacing was 2.0 ± 0.07 μm.
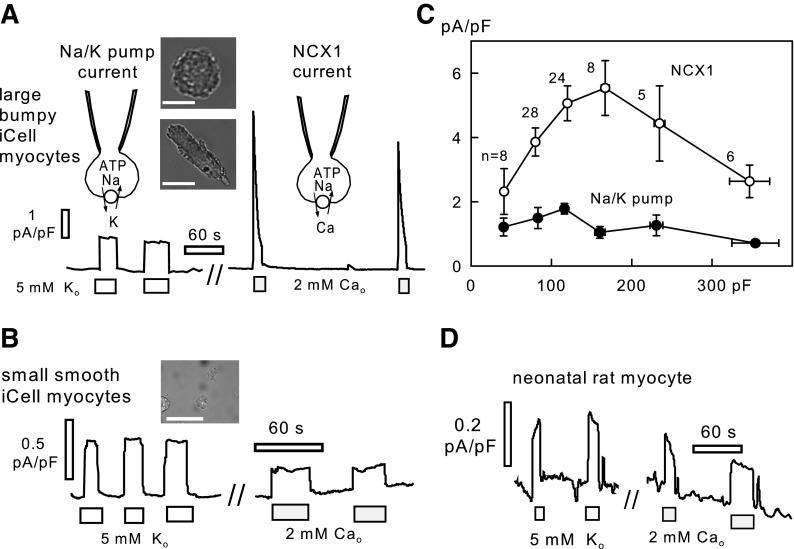
Figure 2, A and B, shows micrographs of iCell Caridiomyocytes after deplating, together with recordings of Na/K pump and Na/Ca exchange currents. In our experience, the largest current densities were recorded in large cells that appeared “bumpy,” presumably a reflection of well-developed myofilaments. As the two micrographs in Fig. 2A illustrate, the appearance of such cells ranges from a rounded form to an elongated form, more reminiscent of an adult myocyte. The micrograph in Fig. 2B shows typical small, smooth cells. With the use of 40 mM cytoplasmic Na, pump currents were activated by substituting 5 mM Na in the extracellular solution for 5 mM K, and NCX1 currents were activated by applying 3 mM Ca in the presence of 0.5 mM EGTA in the extracellular solution. For the “larger” myocytes in this dish (Cms of 100 to 250 pF), Na/K pump currents amounted to 0.98 ± 0.1 pA/pF, and peak Na/Ca exchange currents amounted to 4.5 ± 0.5 pA/pF (n = 10). The ratios of these current magnitudes are consistent with current densities in guinea pig (10, 14) or rabbit cardiomyocytes (1, 4, 37). In contrast, “smaller” iCell Cardiomyocytes (Cms of 40–100 pF) from the same dish had Na/K pump current densities of 0.7 ± 2 pA/pF and peak Na/Ca exchange current densities of 1.8 ± 0.5 pA/pF in this dish (n = 5).
Fig. 2.
Representative Na/K pump and Na/Ca exchange current recordings from iCell Cardiomyocytes and rat neonatal cardiomyocytes at 37°C. A: recordings from a large iCell Cardiomyocyte with well-developed myofilaments. The 2 micrographs illustrate the range of morphologies of large iCell Cardiomyocytes. Scale bars = 50 μm. B: recordings from a round iCell Cardiomyocyte with more fibroblast-like morphology in bright field microscopy. Scale bar = 50 μm. C: average Na/K pump and Na/Ca exchange (NCX1) current densities from 79 recordings, plotted in dependence on the iCell Cardiomyocyte capacitance (Cm). Data points were binned by Cm magnitude as follows: 35–50 pF, 50–100 pF, 100–150 pF, 150–200 pF, 200–250 pF, and 250–400 pF. D: recording of Na/K pump and NCX1 currents in a rat neonatal cardiomyocyte under identical conditions.
Figure 2C shows the current densities of 79 iCell Cardiomyocytes from 10 different dishes, cultured for 2 to 4 wk, which served as control measurements in comparative studies. The current densities are plotted in dependence on Cm, with Cm bins defined in Fig. 2. NCX1 current density falls off below 150 pF, and Na/K pump currents fall off less steeply. Both current densities are also significantly reduced in the myocytes with the largest Cm values. Thus we avoided the largest and smallest cells in routine measurements. When Cm values nevertheless fell out of the norm, the measurements were eliminated from data sets. Accordingly, results for the six largest and eight smallest cells in Fig. 2C were discarded.
As a contrast to iCell Cardiomyocytes, Fig. 2D shows recordings of Na/K pump currents and Na/Ca exchange currents in neonatal rat cardiomyocytes using identical experimental conditions. Pump current densities were invariably <0.5 pA/pF, and we often could not resolve pump currents with confidence in relation to background and leak currents. The example in Fig. 2D is from a culture that displayed relatively large currents (0.21 ± 0.06 pA/pF; n = 6) with average Cm values of 45 ± 4 pF (n = 15). Na/Ca exchange currents never exceeded 1 pA/pF and were usually <0.5 pA/pF. Thus transporter currents in the smaller iCell Myocytes are still more adult-like than transporter currents in neonatal cardiomyocytes.
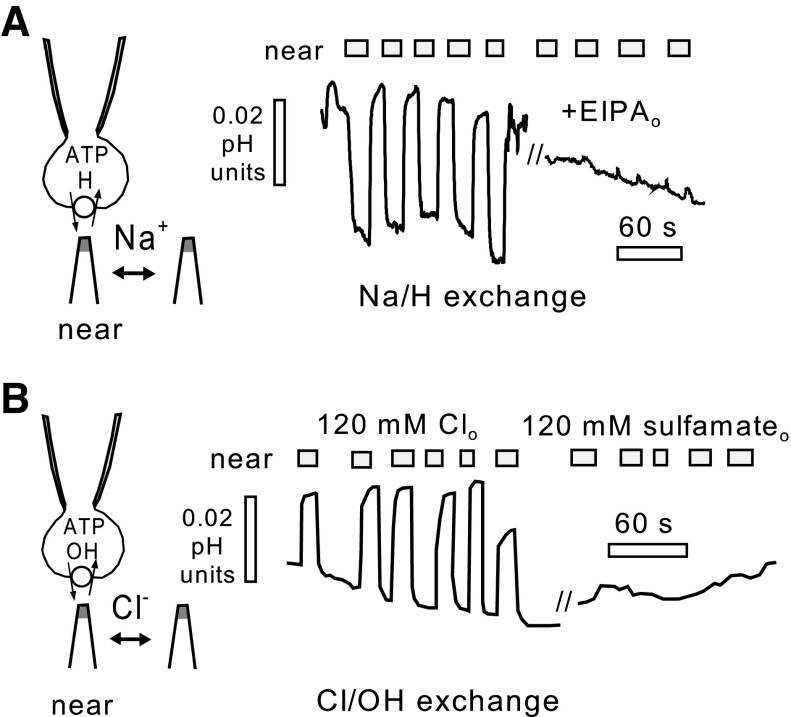
Figure 3 demonstrates the presence of proton transporters in iCells that are consistent with transporters known to exist in adult cardiac myocytes. For these recordings, we employed oscillating micro pH electrodes to detect pH gradients next to cells in the presence of a low extracellular pH buffer concentration (8, 9). As shown in Fig. 3A, Na/H exchange was detected using a cytoplasmic solution set to pH 6.8 with 40 mM HEPES and no Na, together with an extracellular solution set to pH 8.2 (0.1 mM Tris) with 140 mM Na. Upon moving the cell close to the pH electrode, an acidifying pH gradient of 0.03 pH units is detected, and the gradient under these conditions is completely abolished when the extracellular solution is switched to one with 10 μM of the Na/H exchange inhibitor EIPA (21). Under other conditions, however, substantial proton fluxes occur in iCell Cardiomyocytes by mechanisms that we did not detect in the different cell types previously employed with this method (9). Figure 3B shows that, similar to adult cardiac myocytes (29), iCell Cardiomyocytes can generate substantial proton fluxes via Cl/OH exchange. To monitor this flux in isolation, Na-free solutions were employed using K as the major cation. The cytoplasmic solution contains 1 mM Cl with sulfamate as the Cl substitute at pH 7.6, and the extracellular solution contains 140 mM KCl at pH 6.5 [0.1 mM 2-(N-morpholino)ethanesulfonic acid]. In this condition, the proton (or hydroxyl) flux magnitude, recorded as an alkalinizing pH gradient, is similar to the maximal Na/H exchange flux, and the flux is fully blocked by substituting extracellular Cl for sulfamate.
Fig. 3.
Proton transport in iCell Cardiomyocytes resolved by oscillating extracellular pH electrodes in the presence of low (0.1 mM) extracellular pH buffer power. A: Na/H exchange. Outward proton flux is defined by the presence of an extracellular acidification that occurs in the presence of 140 mM extracellular Na at a pH of 8.2 (0.1 mM Tris) with an internal pH of 6.8, and that is blocked by the Na/H exchanger (NHE1) inhibitor EIPA (10 μM). B: Cl/OH exchange. Outward hydroxyl (or inward proton) flux is defined by the presence of an extracellular alkalinization at pH 6.5 [0.1 mM 2-(N-morpholino)ethanesulfonic acid] that occurs in the complete absence of Na on both membrane sides, absence of bicarbonate on both membrane sides, and absence of Cl in the cytoplasm at a pH of 7.6 and that is fully blocked by substituting extracellular Cl for sulfamate.
Figures 4–6 demonstrate that iCell Cardiomyocytes are readily amenable to standard molecular biological approaches to manipulate selected proteins. In Fig. 4, we demonstrate knockdown of the NCX1 Na/Ca exchanger by siRNA transfection. In the Western blot, shown in Fig. 4A, the lanes were arranged to determine NCX1 and actin densities in duplicate for 3-day mock transfections, 3-day scrambled RNAi transfections, 3-day NCX1 RNAi transfections, 5-day mock transfections, 5-day scrambled RNAi transfections, 5-day NCX1 RNAi transfections, and an NCX1 control for BHK cells that highly overexpress NCX1 (23). The duplicate measurements demonstrate >75% loss of NCX1 protein in 3 days and almost complete loss within 5 days with no change of actin as a control protein. Figure 4B demonstrates the specific loss of NCX1 currents in iCell Cardiomyocyte recordings in relation to Na/K pump currents, and Fig. 4C quantifies the functional loss of exchange current over 3 days. Figure 4D shows the time courses of loss of NCX1 protein and current. The best exponential fits give a time constant of 50 h for loss of protein and a time constant of 45 h for loss of exchange currents. Clearly, the average lifetime of exchangers must be >1 day and <3 days, and the results give no evidence for discrepancies between the total protein pool and the “functional” surface membrane pool of NCX1.
Fig. 4.
Knockdown of NCX1 by siRNA transfection in iCell Cardiomyocytes. A: Western blots illustrating the time course and selectivity of NCX1 knockdown with respect to actin. Densities were related to the density of NCX1 bands in lane 5 with scrambled siRNA for 5 days. B: typical Na/K pump and NCX1 current records in large mock-transfected and siRNA-transfected cardiomyocytes. C: average current densities in large siRNA-transfected cardiomyocytes immediately after transfection and after 1 and 3 days, respectively. D: plots of NCX1 densities from Western blots on days 0, 3, and 5, and of NCX1 current densities on days 0, 1, and 3 after siRNA transfection. Best fits to an exponential function yield time constants of 50 and 45 h for loss of protein and current, respectively.
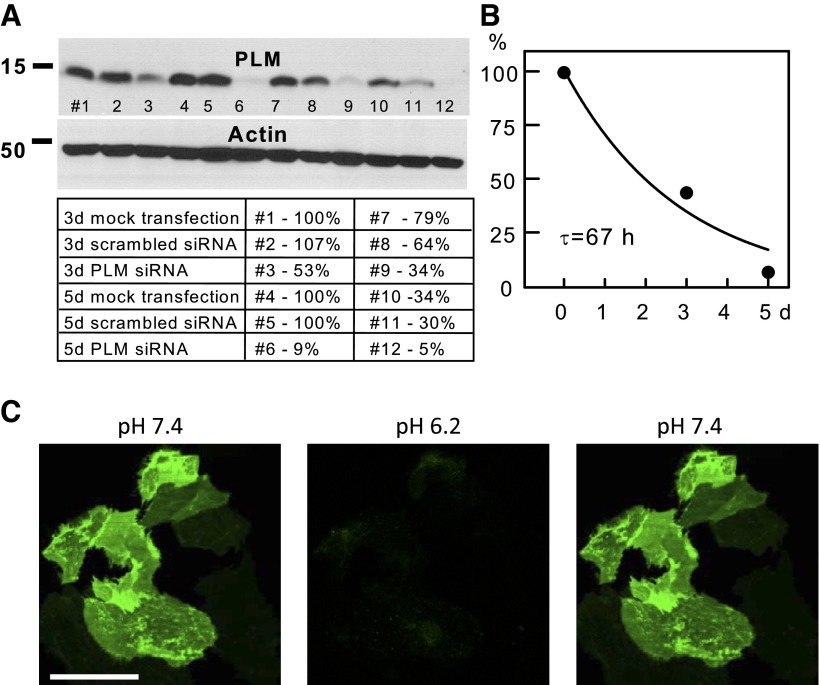
Fig. 6.
Knockdown of phospholemman (PLM) by siRNA transfection in iCell Cardiomyocytes. A: Western blots illustrating the time course and selectivity of PLM knockdown with respect to actin. Densities were related to the density of lane 5 with scrambled siRNA for 5 days. B: Average PLM densities from Western blots at days 0, 3, and 5 after siRNA transfection. The best fit to an exponential function gives a time constant of 67 h for loss of PLM. C: overexpression of a PLM-pHluorin fusion protein with pHluorin being fused to the extracellular N-terminal domain of PLM. Left: image at pH 7.4 shows the usual nonuniform fluorescence pattern suggesting that PLM is located preferentially at sites of cell attachment to the dishes and/or in large sarcolemmal domains. Scale bar = 50 μm. Middle: image illustrates the complete loss of fluorescence that occurs immediately after switching the extracellular pH to 6.2, indicating that fluorescence of the PLM fusion originates entirely from the sarcolemma. Right: image was obtained immediately after switching the extracellular solution back to pH 7.4, further documenting that all fluorescence originates from the sarcolemma.
In Fig. 5, we analyze NCX1 turnover by overexpressing an exchanger fusion that can be covalently tagged on an extracellular Halo domain (19), the domain being fused to the exchanger close to the glycosylation site of the first extracellular segment of the exchanger. Exchangers were labeled with impermeable HaloTag AlexaFluor 488 Ligand (G1002, Promega) at time zero, the extracellular solution was exchanged for solution without tag, and the occurrence of green punctae within cells was quantified at the given time points subsequent to labeling. Representative micrographs of this progression are shown in Fig. 5A over 12 h after labeling cells. Figure 5B quantifies the development of internal punctae. The increase is clearly far from saturation at 12 h, and the best fit to an asymptotic exponential function gives a time constant for NCX1 internalization of 42 h. This time constant is consistent with that for loss of NCX1 protein and current, as described in Fig. 4. Although fluorescence of the Halo-tagged exchangers subsequently decreased, this decrease may arise from any of multiple chemical possibilities unrelated to exchanger degradation (e.g., fluorophore degradation). Therefore, fluorescence changes were not analyzed in detail over longer time periods.
Fig. 5.
Internalization of NCX1-Halo fusion protein after labeling. A: NCX1 with an extracellular Halo fusion near the NH2-terminal glycosylation site was expressed in iCell Cardiomyocytes for 4 days. The Halo tag was then labeled with Alexa488 Halo label and iCells were imaged at fixed intervals for 12 h. Over this time a nearly linear increase of cytoplasmic punctae occurred, indicative of NCX1 internalization. Scale bar = 20 μm. B: plot of the average number of defined nonnuclear punctae. The best fit to an asymptotic exponential function gives a time constant of 42 h for NCX1 loss from the cell surface.
Figure 6 demonstrates knockdown and overexpression of the sarcolemmal regulatory protein PLM (2). The Western blot shown in Fig. 6A documents first that mock transfections result in no change of PLM or actin expression over 5 days. In this blot, there is an apparent loss of protein in lane 10 vs. 4 and lane 5 vs. 11, probably related to inefficient protein transfer. Nevertheless, it is clear that after siRNA of PLM for 3 days loss of PLM amounts to only ∼60%, while protein decreases to negligible levels after siRNA transfection within the 5-day period. As shown in Fig. 6B, the best fit of PLM densities from Western blots gives a time constant of 67 h for the loss of PLM after siRNA transfection. Figure 6C demonstrates overexpression of a PLM fusion with the pH-sensitive green protein pHluorin similar to constructs developed with NCX1 (20). In this case the pHluorin was fused to the extracellular NH2-terminal of PLM. As shown in Fig. 6C, left, the expression of the PLM-pHluorin fusion appears very inhomogeneous, being distinctly concentrated in areas that possibly correspond to membrane attachment zones of the iCells to the coverslip. Regardless of the reason for the inhomogeneous expression, application of an extracellular solution with a pH of 6.2 immediately quenched the entire PLM fluorescence and washout of the acidic solution resulted in an immediate return of fluorescence to the original pattern. This result indicates that the visible PLM-pHluorin fusion protein is located entirely in the surface membrane, and the result gives no evidence for internal PLM pools in this condition.
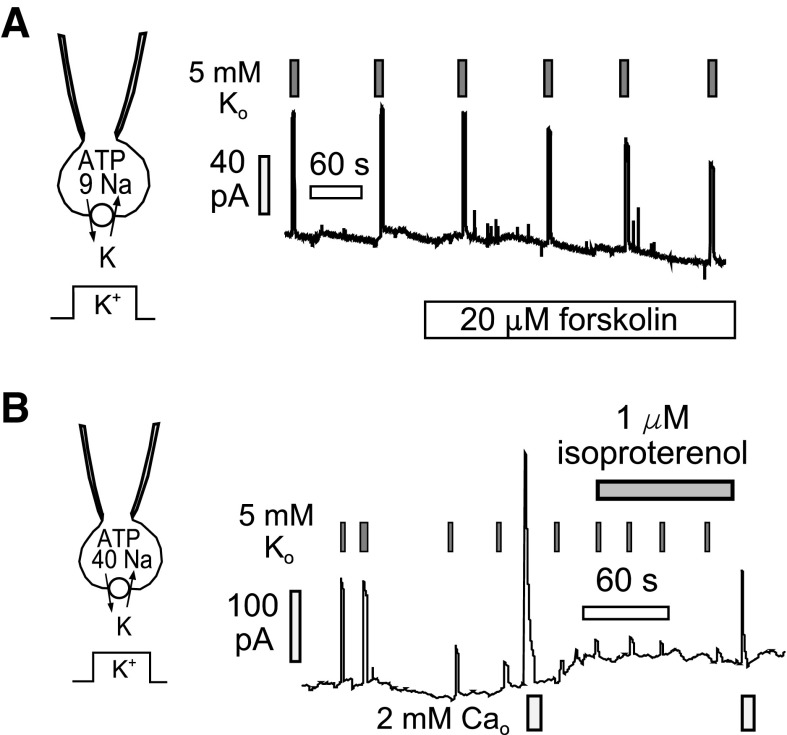
The original goal of our experiments manipulating PLM was to demonstrate and characterize the role of PLM in regulating Na/K pump activity via the cAMP system. However, this has not been possible because we could not demonstrate a stimulatory effect of activating cAMP synthesis on pump activity. In brief, we planned our experiments carefully to be consistent with previous work using Na-sensitive dyes (5). In those experiments, cardiomyocytes were loaded with Na by removing extracellular K and thereby blocking Na/K pumps. Upon activating pumps by application of extracellular K, intracellular Na decreases more rapidly in a mid-range of Na concentrations when cAMP levels are elevated than when they are not. On this basis, activation of the cAMP system should result in an increased pump affinity for cytoplasmic Na and cause a doubling of pump activity in the mid-range of the Na activation curve. As shown in Fig. 7A, we employed a cytoplasmic Na concentration (9 mM), which is submaximal for activation of Na/K pump currents. Pump currents were activated by brief application of 5 mM K to avoid depletion of cytoplasmic Na, and we promoted adenylate cyclase activity by applying either forskolin (20 μM in Fig. 7A) or isoproterenol (0.5 μM). No change of pump current magnitudes was observed with either adenylate cyclase activating agent.
Fig. 7.
Representative experiments intended to define a stimulatory effect of cAMP generation on Na/K pump currents in iCell Cardiomyocytes. A: with the use of a submaximal cytoplasmic Na concentration of 9 mM, pump currents were activated multiple times by applying 5 mM K in exchange for 5 mM Na in the extracellular solution. To avoid depletion of cytoplasmic Na, currents were deactivated by removing extracellular K after 3 s. Application of 20 μM forskolin to activate adenylate cyclases failed to cause any enhancement of pump currents. B: with the use of a maximal cytoplasmic Na concentration of 40 mM, pump currents often underwent a time-dependent rundown over the course of several minutes. We tested therefore whether activation of adenylate cyclase activity by β-adrenergic stimulation might restore pump currents. No restoration was observed, indicating that pump rundown is not caused by dephosphorylation of cAMP kinase-sensitive sites.
As shown in Fig. 7B, we then considered a possibility that pumps might be “preactivated” by a constitutively active cAMP system in iCell Cardiomyocytes. This possibility was consistent with the fact that pump currents often exhibited rundown during experiments. If this rundown reflected dephosphorylation of PLM, activation of cAMP synthesis would be expected to reactivate pump currents. Figure 7B shows an example in which we monitored rundown of both pump currents and exchange current. Pump current ran down by >75% over a few minutes and was not restored by application of the β-adrenergic receptor agonist isoproterenol. Outward NCX1 currents decreased, as usual, during multiple activation episodes, and this decrease was also unaffected by isoproterenol.
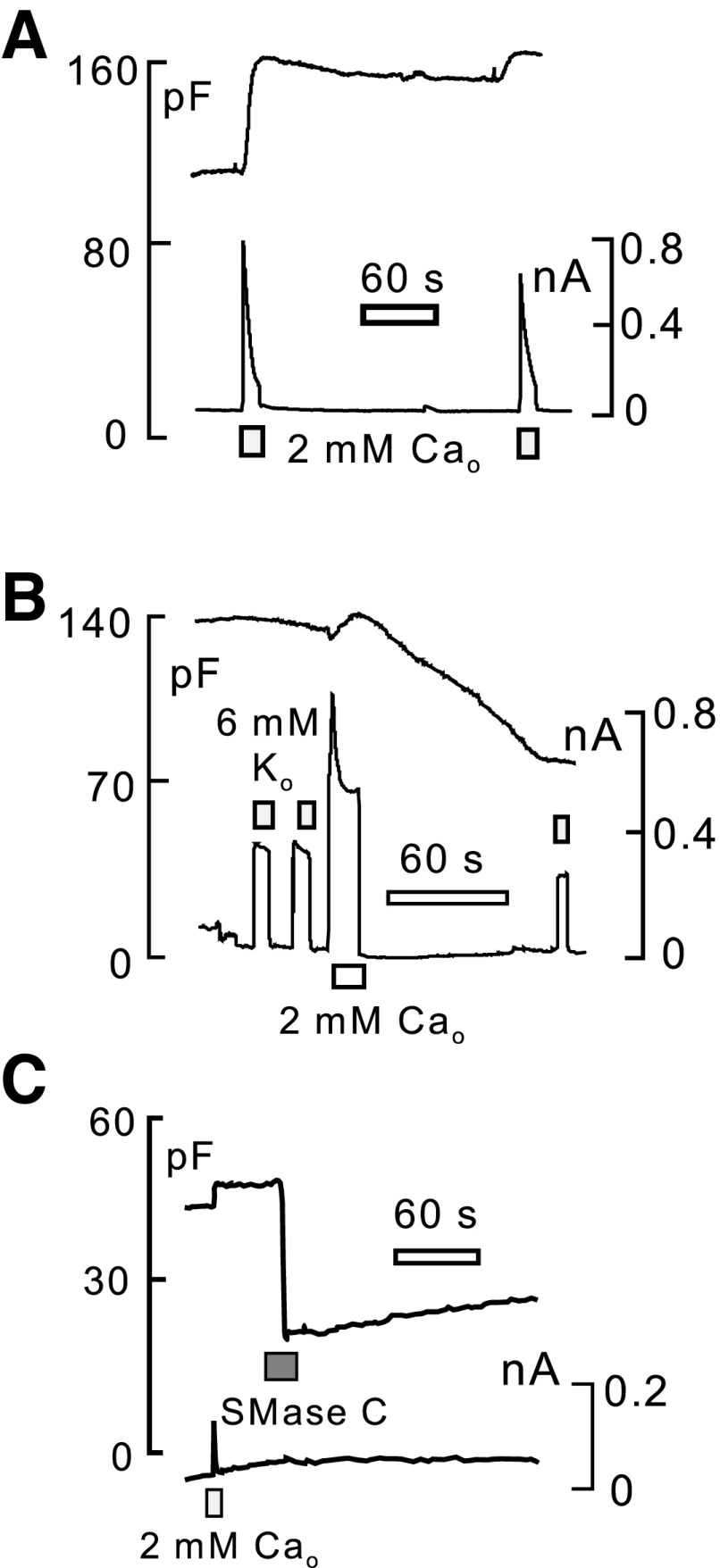
Finally, we used Cm recording to characterize easily interrogated properties of sarcolemma turnover in iCell Cardiomyocytes. We describe in Fig. 8 Cm changes of iCell Cardiomyocytes in response to Ca transients induced by activation of outward Na/Ca exchange current (i.e., in response to Ca influx) and in response to sphingomyelin cleavage by sphingomyelinase C. As shown in Fig. 8A, activation of outward exchange current in the presence of a low cytoplasmic concentration of EGTA (0.5 mM) results in a large increase of the iCell Cardiomyocyte Cm, roughly a 30% increase in this case over 10–15 s. The average increase for 21 measurements was 14 ± 2.2%. This pattern is typical in our experience for fibroblasts (41), and, further in this regard, we have never observed Ca-activated exocytosis of significant magnitude in isolated cardiomyocytes, including adult and neonatal cardiomyocytes from several species (20). Over time, the enhanced Cm decayed very little in most iCell Cardiomyocyte recordings. The exchange current activated by a second application of extracellular Ca was invariably decreased in magnitude, and any additional increase of Cm was relatively small. As shown in Fig. 8B, we sometimes observed that, subsequent to the exocytic phase, Cm decreased over 1–2 min substantially. This pattern, typical for fibroblasts (20), was observed rather frequently when iCell Myocytes had been cultured for >3 wk and never when cells were cultured for <2 wk. In this experiment, we also followed the magnitude of Na/K pump currents to test whether the pump is affected in a selective way by the endocytic response. The pump current decreased roughly in proportion to the loss of Cm. Similar to the exocytic responses just described, we have never observed this pattern in adult cardiomyocytes; rather, endocytosis typically occurs immediately during large Ca transients in adult cardiomyocytes and ceases rather abruptly when Ca influx is terminated (20). Finally, Fig. 8C illustrates that iCell Cardiomyocytes respond to sphyingomyelin cleavage during extracellular application of purified sphingomyelinase C (20) by massive endocytosis that constitutes >50% of the cell surface. Similar responses are observed in adult cardiomyocytes, as well as fibroblasts (20). Thus this result simply documents that the iCell sarcolemma has basic physical properties that are similar to other cell types.
Fig. 8.
Cm recordings of surface membrane turnover in iCell Cardiomyocytes. A: typical exocytic responses observed during activation of maximal outward Na/Ca exchange currents with 2 mM extracellular Ca. Cytoplasmic solution contains 40 mM Na and 0.5 mM EGTA with 0.25 mM Ca to give 0.5 μM free Ca. Extracellular solution is Na free. Upon the initial activation of Ca influx, Cm increases by 30% over 10 s. Decline of Cm after deactivating exchange current is minimal, and a second activation of exchange current results in only a small further increase of Cm. B: typical endocytic response observed in iCell Cardiomyocytes that had been cultured for >3 wk. Activation of Ca influx via Na/Ca exchange results in only a small exocytic response followed by a slow loss of ∼30% of Cm over several minutes after the exchange current is deactivated. C: activation of a massive (>50%) endocytic response by brief (20 s) application of sphingomyelinase C (1 U/ml) purified from Bacillus cereus.
DISCUSSION
We have described in this article measurements of four ion transport activities and Ca-dependent Cm changes in human-induced pluripotent stem cell-derived cardiomyocytes, thereby allowing comparisons of these cells to adult cardiac myocytes, as well as to other cells such as fibroblasts, that can be studied with the same methods. In addition, we have described basic molecular biological manipulations of iCell Cardiomyocytes to access their potential utility to study cellular regulation and turnover of ion transporters. Together, the results document that iCell Cardiomyocytes are an advantageous and promising new cardiac myocyte culture system for a wide range of ion transport studies. The results also allow us to access potential limitations of these cells for some goals. We discuss here both the overriding advantages and certain limitations of iCell Cardiomyocytes in detail.
iCell cardiomyocytes allow both acute and chronic studies of human cardiac transporters.
As illustrated in Fig. 1, the cell selection method, based on the cardiac MYC6 promoter, clearly is successful in generating a cardiomyocyte cell culture with consistent 2-μm myofilament structures and, as described previously (25), with syncytial coupling of cardiomyocytes upon expansion to confluence. As described in Fig. 2, the larger iCell Cardiomyocytes have visible features more similar to mature cardiac myocytes and consistently display Na/K pump and Na/Ca exchange current magnitudes that are equivalent to those of adult guinea pig (10, 14) or rabbit cardiomyocytes (1, 4, 37). This outcome contrasts with cultured neonatal cardiomyocytes, which, as described in Fig. 2C, have much lower Na transport activities in spite of the fact that they beat at rates (40–100/min; e.g., Ref. 24) that are comparable to those of iCell Cardiomyocytes (25). Presumably for this reason, our literature searches do not reveal any published study of Na/K pump currents in neonatal cardiac myocytes. While the implications of low ion transport capacities for neonatal cardiomyocytes are enigmatic at present, the more adult-like transport function of iCell Cardiomyocytes clearly marks them as a more advantageous cell culture system for future studies. As shown in Fig. 3, iCell Cardiomyocytes display high activities of two pH regulating systems that are characteristic of adult cardiomyocytes, Na/H exchange activity, and Cl/OH exchange (29). As illustrated in Figs. 4–6, the iCell Cardiomyocyte culture system is readily amenable to the application of standard molecular biological techniques to knockdown and overexpress individual proteins that mediate and/or regulate cardiac ion transport. Specifically, we have demonstrated nearly complete knockdown of NCX1 and PLM by siRNA, and we have demonstrated overexpression of two fluoroescent fusion proteins. These results together clearly open the way to a wide range of acute and long-term studies of human cardiac ion transport not readily possible in cardiomyocyte culture systems to date.
Membrane protein turnover rates and pathways in iCell cardiomyocytes.
In Figs. 4–6, we have presented experiments documenting that iCell Cardiomyocytes can be used to characterize the turnover of ion transporters and ion transport regulatory proteins. Figure 4 documents that siRNA transfection to knockdown the cardiac Na/Ca exchanger NCX1 results in nearly complete loss of NCX1 protein in 3–5 days, and that NCX1 function declines selectively with a similar time course. Importantly, Na/Ca exchange currents do not decline more rapidly than NCX1 protein; NCX1 current is still 65% of normal after 24 h. This result contrasts strongly to a study using antisense nucleotides to knockdown NCX1 in neonatal cardiac myocytes (6), whereby NCX1 function was lost much more rapidly than NCX1 protein. To test further whether plasmalemmal NCX1 might turn over faster than total NCX1 protein in iCell Cardiomyocytes, we overexpressed an NCX1-Halo fusion that can be labeled from the extracellular side to follow loss of NCX1 from the cell surface. This approach, described in Fig. 5, also gave no evidence that NCX1 transporters localized to the cell surface turn over faster than the total NCX1 protein pool. In contrast, the appearance of punctae in the cytoplasm, indicative of internalized NCX1 protein, occurred almost linearly over a 12-h period, and the extrapolated time constant of turnover is in the same 40- to 50-h range that we obtain for loss of total NCX1 protein and NCX1 function. That the relatively slow turnover of NCX1 in iCell Cardiomyocytes is not atypical is verified in Fig. 6 by analysis of PLM knockdown with siRNA. The loss of PLM is definitively only partial after 3 days, and it becomes nearly complete within 5 days. Thus the turnover of PLM in iCell Cardiomyocytes appears to be even longer than that of NCX1, the minimum time constant being >60 h.
Finally, we demonstrated in Fig. 6C the use of an extracellular pHluorin fusion with PLM to determine localization of an overexpressed membrane protein in iCell Cardiomyocytes. Fluorescence micrographs of the fusion protein reveal a complex pattern that alone would not allow conclusions about the cellular localization of overexpressed protein. However, the immediate loss of fluorescence upon acidifying the extracellular space to pH 6.2 demonstrates that the entire visible pHluorin-PLM fusion pool is indeed localized to the sarcolemma. The reasons why PLM expression in the cell surface is inhomogeneous are not clear at this time. Prominent PLM domains might reflect, for example, places of cardiomyocyte attachment to the coverslips, presumably dependent on integrins and cytoskeleton, or the domains might reflect other types of lateral membrane inhomogeneities, for example, the presence or more ordered versus less ordered macroscopic membrane domains. Clearly, iCell Cardiomyocytes provide a powerful new model to address questions about sarcolemmal protein localization and transporter interactions with protein networks in relation to membrane domains.
The PLM conundrum.
It is well established that PLM is a regulatory subunit of the cardiac Na/K pump, and it is widely appreciated that PLM inhibition of Na/K pumps is relieved by phosphorylation via cAMP-dependent protein kinases (PKAs) and protein kinase Cs (PKCs) (5). Nevertheless, the stimulation of Na/K pump activity by protein kinase activation is not always evident, especially not in patch-clamp experiments. As noted in results, relatively large effects of cAMP kinase activation, apparent in experiments using Na dyes to analyze pump activity (5), are consistent with cAMP kinase-dependent PLM phosphorylation increasing the apparent affinity of Na/K pumps for cytoplasmic Na. Nevertheless, using patch clamp to determine pump activity as a membrane current, multiple groups have found no evident effect of PKAs under conditions that seem directly relevant to the results with Na dyes (15, 26). It was our hope that iCell Cardiomyocytes would allow us to clearly define conditions in which activation of PKAs causes substantial stimulation of pump activity. However, we tested a range of experimental conditions using both forskolin and isoproterenol to activate adenylate cyclase and, as illustrated in Fig. 7, were unsuccessful. In addition, we eliminated a possibility that pump rundown reflects loss of PKA-dependent pump stimulation. Isoproterenol did not reactivate Na/K pump activities subsequent to pump rundown. In short, we have not resolved the long-standing conundrum that some groups observe PKA-dependent activation of cardiac Na/K pump activity, while others do not.
iCell cardiomyocyte advantages in relation to limitations for transport studies.
As documented above, the list of experimental opportunities afforded by iCell Cardiomyocytes to study cardiac ion transport is substantial: iCell Cardiomyocytes have human adult-like expression of major cardiac ion transporters, they allow long-term molecular manipulation of individual proteins and regulatory factors, and they allow analysis of transporter and regulatory protein turnover. In apposition to these benchmarks, we have tentatively encountered two limitations. First, iCell Cardiomyocytes clearly exist in a range of phenotypic states. For example, electrophysiological characterization of iCell Cardiomyocytes and other stem cell derived cardiomyocytes has shown the presence of atrial-, nodal, and ventricular-like cardiomyocytes (25, 30). Immunocytochemical characterization has also shown differential expression of cardiac markers myosin light chain (MLC)2v and MLC2a and that the expression pattern of these markers can change over time in culture (16). In our experience, clear differences in cellular morphology and membrane surface area of trypsinized iCell Cardiomyocytes correspond well to differences in transporter expression. Small cells with poorly developed myofilaments have lower NCX1 current densities and relatively low Na/K pump current densities. Current densities also are decreased in the fractions of iCell Cardiomyocytes with the largest Cms of >150 pF. The reasons for these dependencies on myocyte size are unknown at this time. Hypertrophy of cardiac myocytes can be demonstrated to cause a decrease of Na/K pump current density caused by local Na depletion during pump current measurements, rather than a genuine decrease of pump capacity (36). It remains to be tested whether a similar mechanism affects Na/K pump current measurements in iCell Cardiomyocytes in dependence on cell size. For NCX1 currents, it remains also to be tested whether the decrease of current densities in larger cells might be the result of low Ca buffering (0.5 mM EGTA) employed in the present experiments. As with adult cardiomyocytes, it can be difficult to be sure that only one cell is voltage clamped. It is readily possible that recordings with large Cms reflect the activity of two or more cells coupled by gap junctions, thereby introducing diffusional complexities. The major implication for future experiments is that iCell Cardiomyocytes must be selected carefully to be comparable. This can best be accomplished objectively by performing comparative experiments in a blinded fashion.
Second, the Cm responses of iCell Cardiomyocytes to Ca transients, as described in Fig. 8, are similar to responses that we commonly observed in fibroblasts and HEK293 cells (20, 41), rather than adult cardiomyocytes (20). Many explanations of these results are possible at this time. One possibility is that the growth of cardiomyocytes on dishes, which promotes a flattened morphology, versus within a three-dimensional matrix in situ affects membrane trafficking and recycling mechanisms. Alternatively, acutely isolated adult cardiac myocytes may have significantly altered membrane cycling properties as a result of isolation procedures, and their function in these types of experiments may not be representative of cardiomyocytes in situ. A third possibility, which cannot be eliminated at this time, is that iCell Cardiomyocytes might retain some fibroblast-like properties that affect how membrane is trafficked and recycles.
In summary, the potential limitations of iCell Cardiomyocytes for studies of cardiac ion transporters and their regulation are significant. However, these limitations also raise new possibilities to address important open questions about the long-term regulation of cardiac ion transporters and the function of the cardiac secretory pathway, potentially in relation to cardiomyocyte differentiation. Beyond reasonable doubt, iCell Cardiomyocytes allow a wide range of new cellular and molecular experimentation, not previously possible, to address the molecular function and regulation of cardiac ion transporters.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-067942 and HL-513225 (to D. W. Hilgemann).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.F., F.-M.L., M.-J.L., O.W.M., H.-R.W., and D.W.H. conception and design of research; M.F., F.-M.L., M.-J.L., H.-R.W., and D.W.H. performed experiments; M.F., F.-M.L., M.-J.L., H.-R.W., and D.W.H. analyzed data; M.F., F.-M.L., M.-J.L., O.W.M., and D.W.H. interpreted results of experiments; M.F., F.-M.L., M.-J.L., and D.W.H. prepared figures; M.F. and D.W.H. drafted manuscript; M.F. and D.W.H. edited and revised manuscript; M.F., F.-M.L., M.-J.L., O.W.M., H.-R.W., and D.W.H. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Blake Anson (Cellular Dynamics International) for critical discussions and helpful advice.
REFERENCES
- 1.Barman P, Choisy SC, Hancox JC, James AF. β-Adrenoceptor/PKA-stimulation, Na(+)-Ca(2+) exchange and PKA-activated Cl(-) currents in rabbit cardiomyocytes: a conundrum. Cell Calcium 49: 233–239, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell JR, Kennington E, Fuller W, Dighe K, Donoghue P, Clark JE, Jia LG, Tucker AL, Moorman JR, Marber MS, Eaton P, Dunn MJ, Shattock MJ. Characterization of the phospholemman knockout mouse heart: depressed left ventricular function with increased Na-K-ATPase activity. Am J Physiol Heart Circ Physiol 294: H613–H621, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Boerma M, van der Wees CG, Wondergem J, van der Laarse A, Persoon M, van Zeeland AA, Mullenders LH. Separation of neonatal rat ventricular myocytes and non-myocytes by centrifugal elutriation. Pflügers Arch 444: 452–456, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Convery MK, Levi AJ, Khananshvili D, Hancox JC. Actions of myristyl-FRCRCFa, a cell-permeant blocker of the cardiac sarcolemmal Na-Ca exchanger, tested in rabbit ventricular myocytes. Pflügers Arch 436: 581–590, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Despa S, Bossuyt J, Han F, Ginsburg KS, Jia LG, Kutchai H, Tucker AL, Bers DM. Phospholemman-phosphorylation mediates the beta-adrenergic effects on Na/K pump function in cardiac myocytes. Circ Res 97: 252–259, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Egger M, Porzig H, Niggli E, Schwaller B. Rapid turnover of the “functional” Na(+)-Ca2+ exchanger in cardiac myocytes revealed by an antisense oligodeoxynucleotide approach. Cell Calcium 37: 233–243, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Foldes G, Mioulane M. High-content imaging and analysis of pluripotent stem cell-derived cardiomyocytes. Methods Mol Biol 2013 May 3 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Fuster D, Moe OW, Hilgemann DW. Lipid- and mechanosensitivities of sodium/hydrogen exchangers analyzed by electrical methods. Proc Natl Acad Sci USA 101: 10482–10487, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuster D, Moe OW, Hilgemann DW. Steady-state function of the ubiquitous mammalian Na/H exchanger (NHE1) in relation to dimer coupling models with 2Na/2H stoichiometry. J Gen Physiol 132: 465–480, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gadsby DC, Nakao M. Steady-state current-voltage relationship of the Na/K pump in guinea pig ventricular myocytes. J Gen Physiol 94: 511–537, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo J, Massaeli H, Xu J, Jia Z, Wigle JT, Mesaeli N, Zhang S. Extracellular K+ concentration controls cell surface density of IKr in rabbit hearts and of the HERG channel in human cell lines. J Clin Invest 119: 2745–2757, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haworth RA. Use of isolated adult myocytes to evaluate cardiotoxicity. II. Preparation and properties. Toxicol Pathol 18: 521–530, 1990 [PubMed] [Google Scholar]
- 13.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res 93: 32–39, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Hilgemann DW, Collins A. Mechanism of cardiac Na(+)-Ca2+ exchange current stimulation by MgATP: possible involvement of aminophospholipid translocase. J Physiol 454: 59–82, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishizuka N, Berlin JR. Beta-adrenergic stimulation does not regulate Na pump function in voltage-clamped ventricular myocytes of the rat heart. Pflügers Arch 424: 361–363, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Kamakura T, Makiyama T, Sasaki K, Yoshida Y, Wuriyanghai Y, Chen J, Hattori T, Ohno S, Kita T, Horie M, Yamanaka S, Kimura T. Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circ J 77: 1307–1314, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Kammermeier H, Rose H. Are isolated cardiomyocytes a suitable experimental model in all lines of investigation in basic cardiology? Basic Res Cardiol 83: 343–349, 1988 [DOI] [PubMed] [Google Scholar]
- 18.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, Livne E, Binah O, Itskovitz-Eldor J, Gepstein L. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest 108: 407–414, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kovalenko EI, Ranjbar S, Jasenosky LD, Goldfeld AE, Vorobjev IA, Barteneva NS. The use of HaloTag-based technology in flow and laser scanning cytometry analysis of live and fixed cells. BMC Res Notes 4: 340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lariccia V, Fine M, Magi S, Lin MJ, Yaradanakul A, Llaguno MC, Hilgemann DW. Massive calcium-activated endocytosis without involvement of classical endocytic proteins. J Gen Physiol 137: 111–132, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lazdunski M, Frelin C, Vigne P. The sodium/hydrogen exchange system in cardiac cells: its biochemical and pharmacological properties and its role in regulating internal concentrations of sodium and internal pH. J Mol Cell Cardiol 17: 1029–1042, 1985 [DOI] [PubMed] [Google Scholar]
- 22.Lieberman M, Roggeveen AE, Purdy JE, Johnson EA. Synthetic strands of cardiac muscle: growth and physiological implication. Science 175: 909–911, 1972 [DOI] [PubMed] [Google Scholar]
- 23.Linck B, Qiu Z, He Z, Tong Q, Hilgemann DW, Philipson KD. Functional comparison of the three isoforms of the Na+/Ca2+ exchanger (NCX1, NCX2, NCX3). Am J Physiol Cell Physiol 274: C415–C423, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Lorenzen-Schmidt I, Schmid-Schonbein GW, Giles WR, McCulloch AD, Chien S, Omens JH. Chronotropic response of cultured neonatal rat ventricular myocytes to short-term fluid shear. Cell Biochem Biophys 46: 113–122, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J, Guo L, Fiene SJ, Anson BD, Thomson JA, Kamp TJ, Kolaja KL, Swanson BJ, January CT. High purity human induced pluripotent stem cell (hiPSC) derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol 301: H2006–H2017, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Main MJ, Grantham CJ, Cannell MB. Changes in subsarcolemmal sodium concentration measured by Na-Ca exchanger activity during Na-pump inhibition and beta-adrenergic stimulation in guinea-pig ventricular myocytes. Pflügers Arch 435: 112–118, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Nag AC, Cheng M. Adult mammalian cardiac muscle cells in culture. Tissue Cell 13: 515–523, 1981 [DOI] [PubMed] [Google Scholar]
- 28.Nag AC, Cheng M, Fischman DA, Zak R. Long-term cell culture of adult mammalian cardiac myocytes: electron microscopic and immunofluorescent analyses of myofibrillar structure. J Mol Cell Cardiol 15: 301–317, 1983 [DOI] [PubMed] [Google Scholar]
- 29.Niederer SA, Swietach P, Wilson DA, Smith NP, Vaughan-Jones RD. Measuring and modeling chloride-hydroxyl exchange in the Guinea-pig ventricular myocyte. Biophys J 94: 2385–2403, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peng S, Lacerda AE, Kirsch GE, Brown AM, Bruening-Wright A. The action potential and comparative pharmacology of stem cell-derived human cardiomyocytes. J Pharmacol Toxicol Methods 61: 277–286, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Sambrano GR, Fraser I, Han H, Ni Y, O'Connell T, Yan Z, Stull JT. Navigating the signalling network in mouse cardiac myocytes. Nature 420: 712–714, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Schwartz P, Piper HM, Spahr R, Spieckermann PG. Adaptation phenomena of adult cardiac myocytes in culture. Basic Res Cardiol 80, Suppl 2: 181–185, 1985 [PubMed] [Google Scholar]
- 33.Sinnecker D, Goedel A, Laugwitz KL, Moretti A. Induced pluripotent stem cell-derived cardiomyocytes: a versatile tool for arrhythmia research. Circ Res 112: 961–968, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Sperelakis N. Cultured heart cell reaggregate model for studying cardiac toxicology. Environ Health Perspect 26: 243–267, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sybers HD, Ingwall J, DeLuca M. Autophagy in cardiac myocytes. Recent Adv Stud Card Struct Metab 12: 453–463, 1976 [PubMed] [Google Scholar]
- 36.Verdonck F, Volders PG, Vos MA, Sipido KR. Increased Na+ concentration and altered Na/K pump activity in hypertrophied canine ventricular cells. Cardiovasc Res 57: 1035–1043, 2003 [DOI] [PubMed] [Google Scholar]
- 37.White CN, Figtree GA, Liu CC, Garcia A, Hamilton EJ, Chia KK, Rasmussen HH. Angiotensin II inhibits the Na+-K+ pump via PKC-dependent activation of NADPH oxidase. Am J Physiol Cell Physiol 296: C693–C700, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453: 524–528, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Yang Z, Murray KT. Ionic mechanisms of pacemaker activity in spontaneously contracting atrial HL-1 cells. J Cardiovasc Pharmacol 57: 28–36, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaradanakul A, Feng S, Shen C, Lariccia V, Lin MJ, Yang J, Kang TM, Dong P, Yin HL, Albanesi JP, Hilgemann DW. Dual control of cardiac Na+ Ca2+ exchange by PIP(2): electrophysiological analysis of direct and indirect mechanisms. J Physiol 582: 991–1010, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yaradanakul A, Wang TM, Lariccia V, Lin MJ, Shen C, Liu X, Hilgemann DW. Massive Ca-induced membrane fusion and phospholipid changes triggered by reverse Na/Ca exchange in BHK fibroblasts. J Gen Physiol 132: 29–50, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zwi-Dantsis L, Gepstein L. Induced pluripotent stem cells for cardiac repair. Cell Mol Life Sci 69: 3285–3299, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]