Abstract
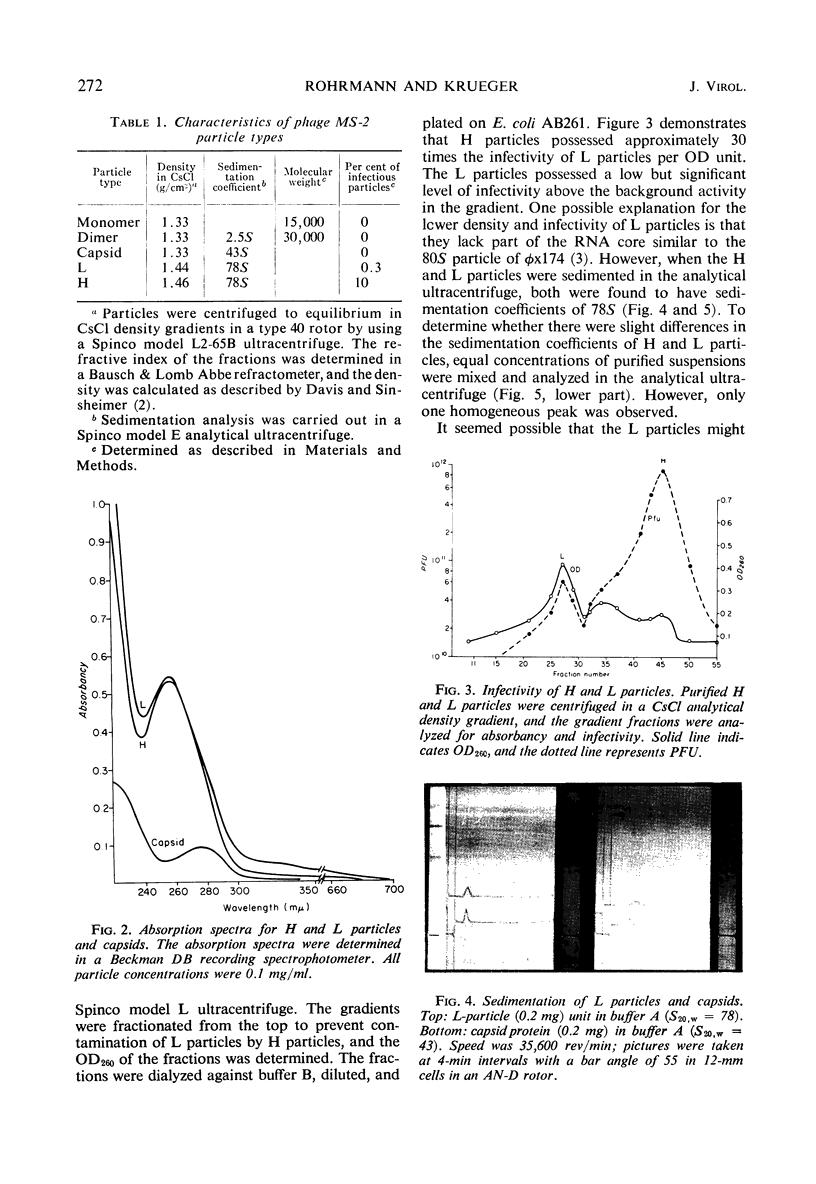
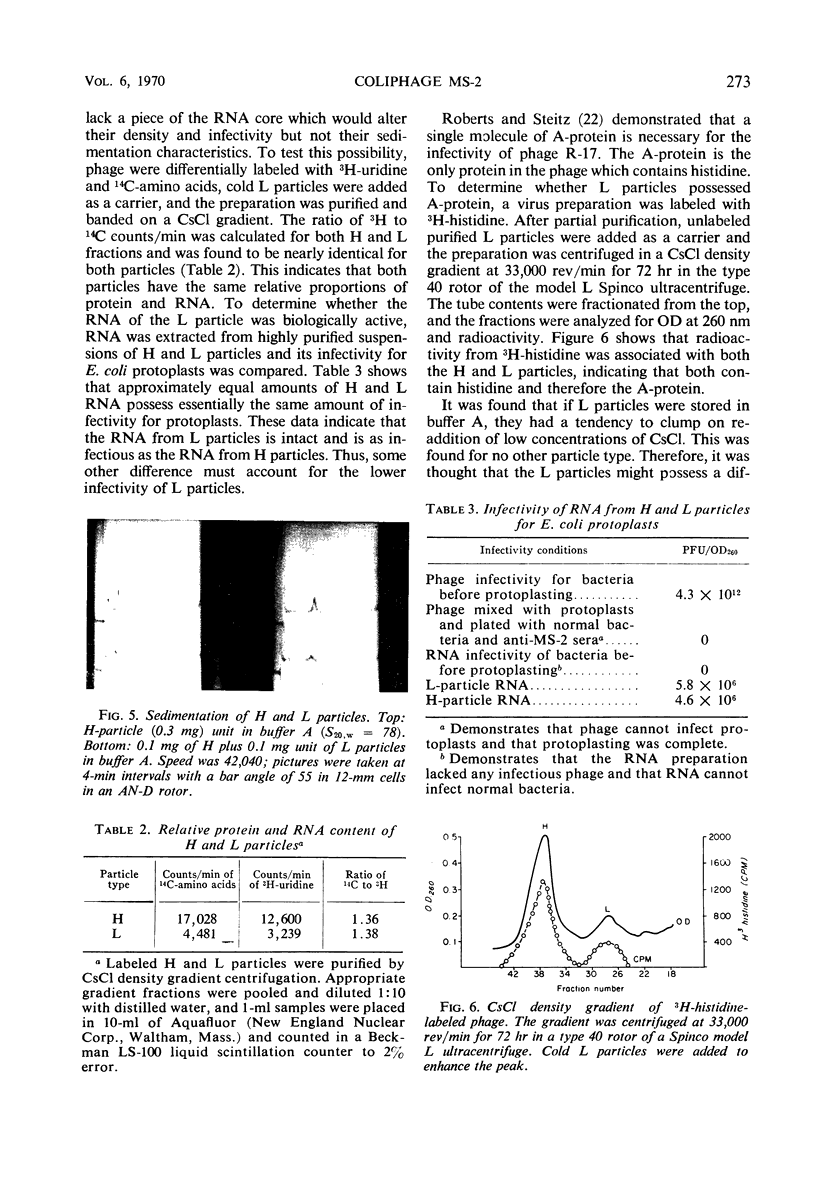
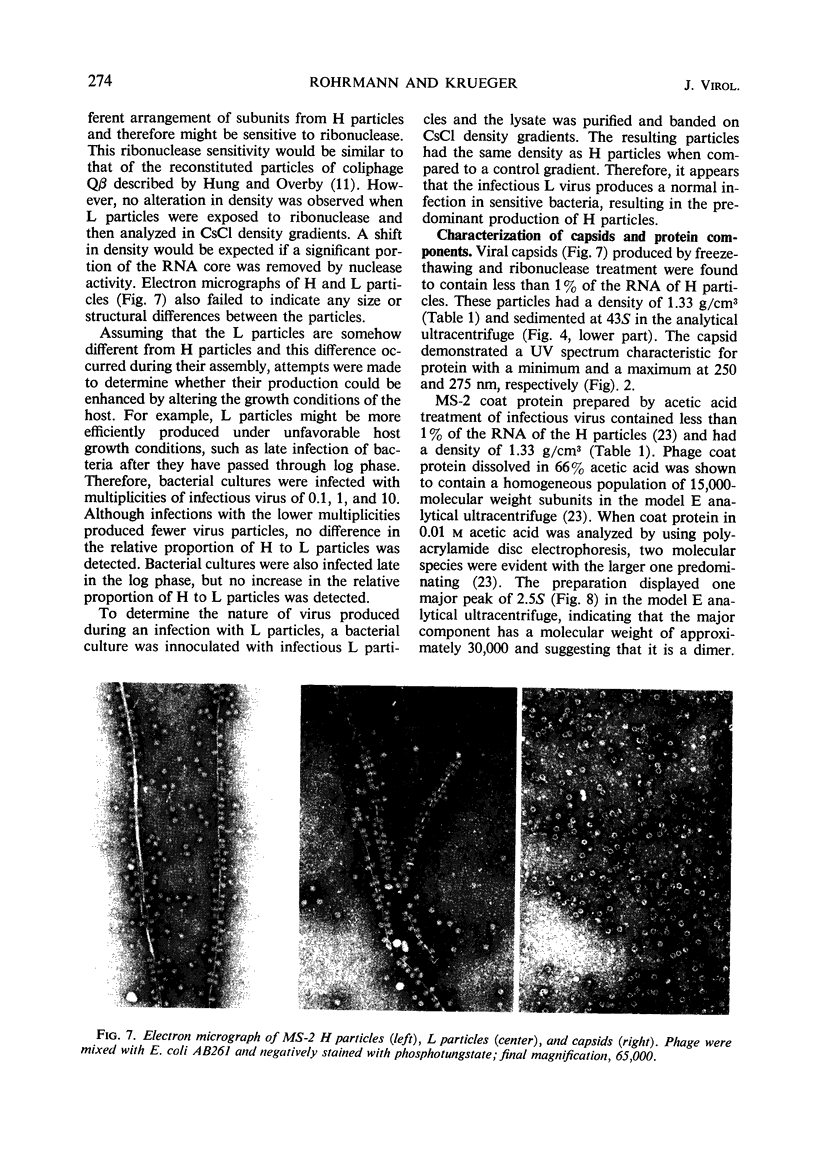
H (heavy) and L (light) MS-2 particles differ in density, absorption spectrum, and infectivity. Studies on their sedimentation, ribonucleic acid (RNA) content and infectivity, appearance under the electron microscope, ribonuclease sensitivity, and A-protein content failed to demonstrate any difference between the two particle types. Studies on the size, RNA content, and density of the capsid and two smaller coat protein components were also conducted. The antigenic relatedness of five different viral and subviral particles of MS-2 were studied by using immunodiffusion and neutralization. Capsids and the H and L viral particles were shown to be antigenically related, whereas the coat protein monomers and dimers were shown to be unrelated to the higher-molecular-weight particles.
Full text
PDF


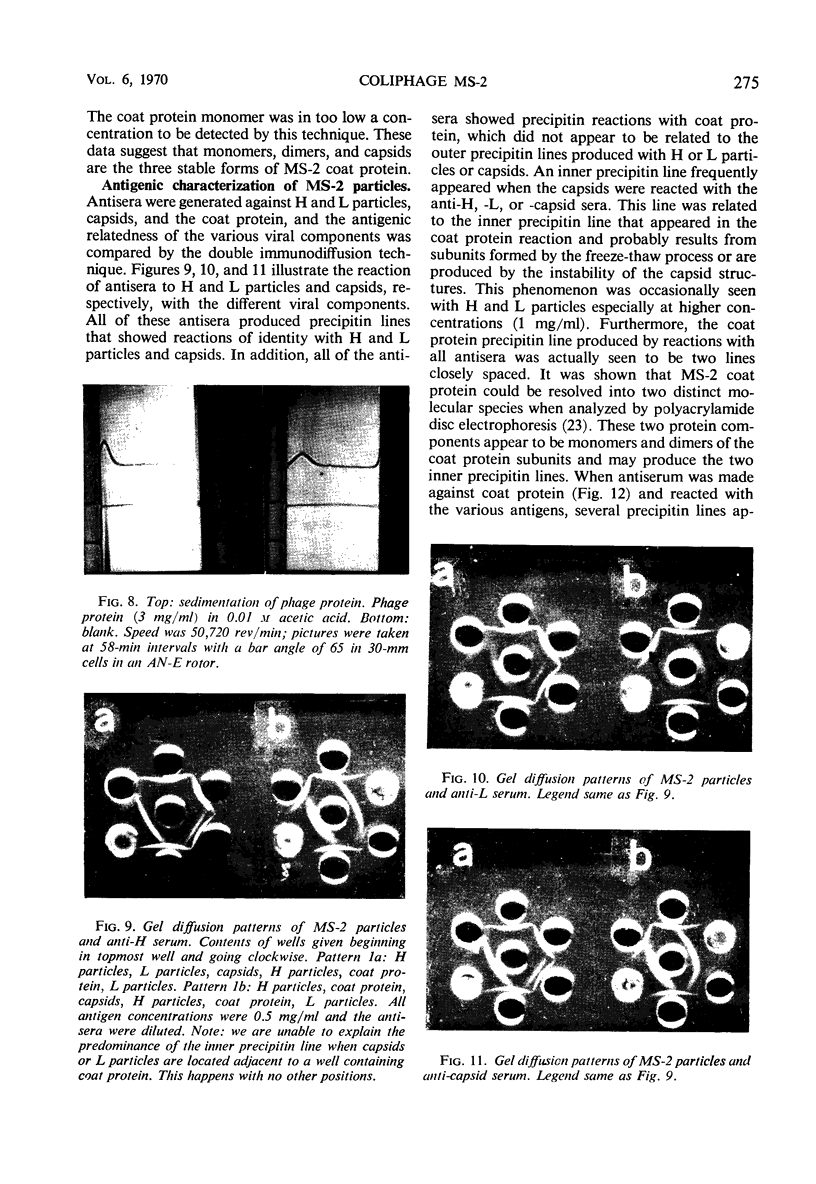
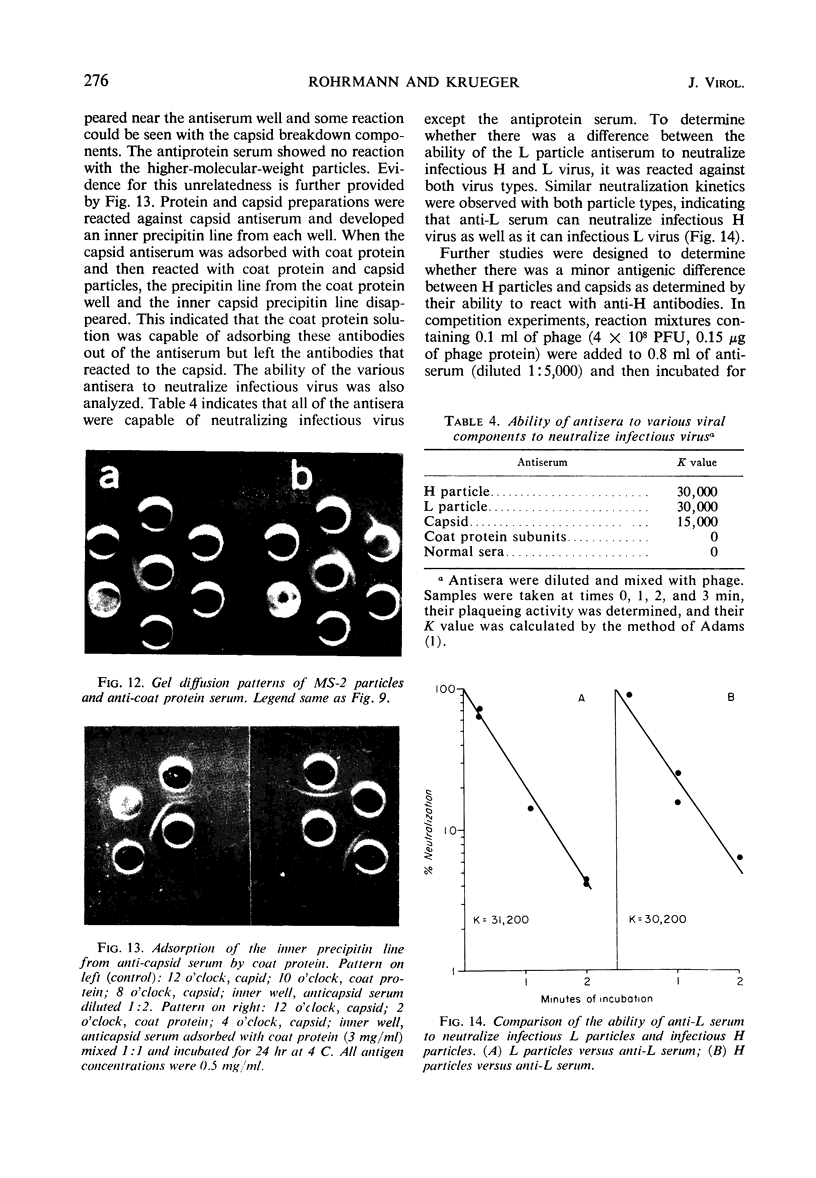
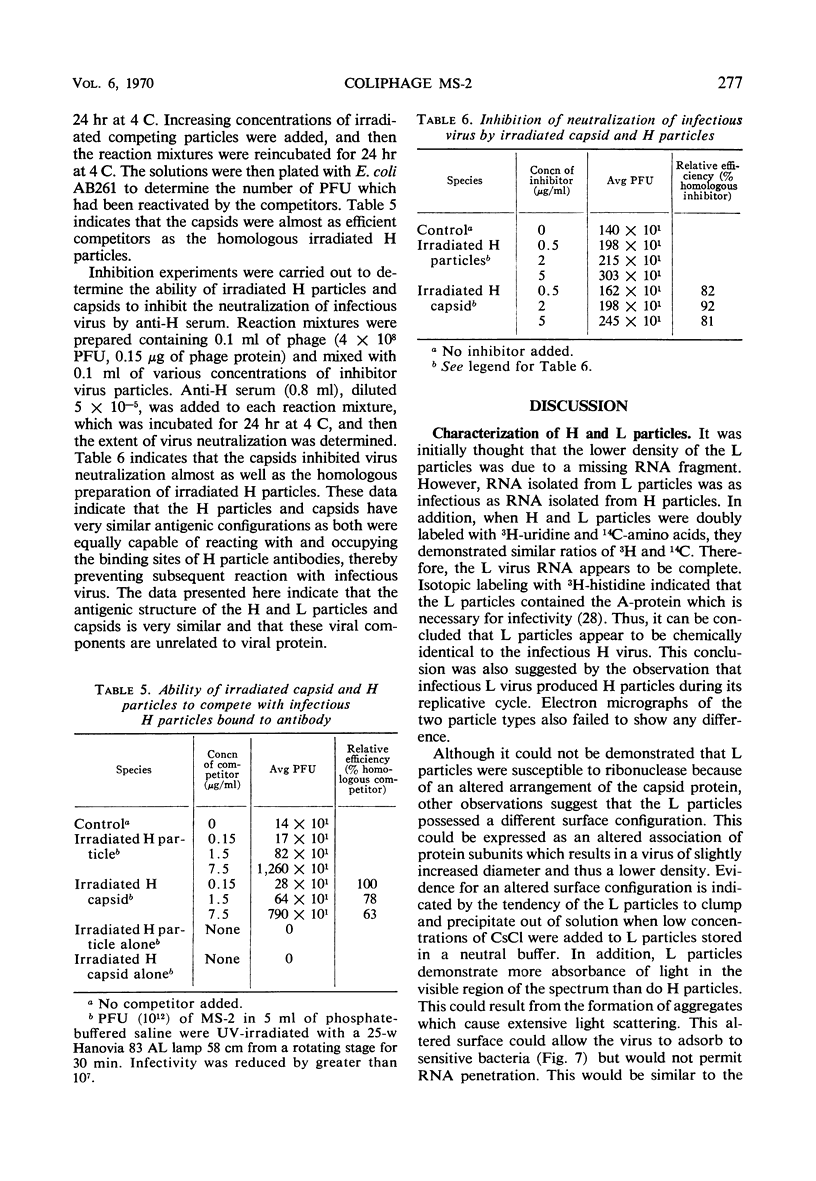


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS J. E., SINSHEIMER R. L. The replication of bacteriophage MS2. 1. Transfer of parental nucleic acid to progeny phage. J Mol Biol. 1963 Mar;6:203–207. doi: 10.1016/s0022-2836(63)80069-8. [DOI] [PubMed] [Google Scholar]
- Enger M. D., Kaesberg P. Comparative studies of the coat proteins of R-17 and M-12 bacteriophages. J Mol Biol. 1965 Aug;13(1):260–268. doi: 10.1016/s0022-2836(65)80095-x. [DOI] [PubMed] [Google Scholar]
- FRASER D., JERREL E. A. The amino acid composition of T3 bacteriophage. J Biol Chem. 1953 Nov;205(1):291–295. [PubMed] [Google Scholar]
- GESTELAND R. F., BOEDTKER H. SOME PHYSICAL PROPERTIES OF BACTERIOPHAGE R17 AND ITS RIBONUCLEIC ACID. J Mol Biol. 1964 Apr;8:496–507. doi: 10.1016/s0022-2836(64)80007-3. [DOI] [PubMed] [Google Scholar]
- HANLON S., LAMERS K., LAUTERBACH G., JOHNSON R., SCHACHMAN H. K. Ultracentrifuge studies with absorption optics. I. An automatic photoelectric scanning absorption system. Arch Biochem Biophys. 1962 Oct;99:157–174. doi: 10.1016/0003-9861(62)90258-8. [DOI] [PubMed] [Google Scholar]
- Herrmann R., Schubert D., Rudolph U. Self-assessment of protein subunits from bacteriophage fr. Biochem Biophys Res Commun. 1968 Mar 12;30(5):576–581. doi: 10.1016/0006-291x(68)90092-2. [DOI] [PubMed] [Google Scholar]
- Hohn T. The assembly of protein particles of the RNA bacteriophage fr in absence of RNA. Biochem Biophys Res Commun. 1969 Jul 7;36(1):7–17. doi: 10.1016/0006-291x(69)90641-x. [DOI] [PubMed] [Google Scholar]
- Hung P. P., Overby L. R. The reconstitution of infective bacteriophage Q beta. Biochemistry. 1969 Mar;8(3):820–828. doi: 10.1021/bi00831a009. [DOI] [PubMed] [Google Scholar]
- Konigsberg W., Weber K., Notani G., Zinder N. The isolation and characterization of the tryptic peptides from the f2 bacteriophage coat protein. J Biol Chem. 1966 Jun 10;241(11):2579–2588. [PubMed] [Google Scholar]
- Krueger R. G. Serological relatedness of the ribonucleic acid-containing coliphages. J Virol. 1969 Nov;4(5):567–573. doi: 10.1128/jvi.4.5.567-573.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger R. G. The effect of Streptomycin on antibody synthesis in vitro. Proc Natl Acad Sci U S A. 1965 Jul;54(1):144–152. doi: 10.1073/pnas.54.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMERS K., PUTNEY F., STEINBERG I. Z., SCHACHMAN H. K. ULTRACENTRIFUGE STUDIES WITH ABSORPTION OPTICS. 3. A SPLIT-BEAM PHOTOELECTRIC, SCANNING ABSORPTION SYSTEM. Arch Biochem Biophys. 1963 Dec;103:379–400. doi: 10.1016/0003-9861(63)90428-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Overby L. R., Barlow G. H., Doi R. H., Jacob M., Spiegelman S. Comparison of two serologically distinct ribonucleic acid bacteriophages. I. Properties of the viral particles. J Bacteriol. 1966 Jan;91(1):442–448. doi: 10.1128/jb.91.1.442-448.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARANCHYCH W. Assay of infectious RNA from bacteriophage R 17. Biochem Biophys Res Commun. 1963 Apr 2;11:28–33. doi: 10.1016/0006-291x(63)90022-6. [DOI] [PubMed] [Google Scholar]
- RAPPAPORT I., SIEGEL A., HASELKORN R. INFLUENCE OF THE STATE OF SUBUNIT AGGREGATION ON THE ANTIGENIC SPECIFICITY OF TMV AND TYMV. Virology. 1965 Feb;25:325–328. doi: 10.1016/0042-6822(65)90211-4. [DOI] [PubMed] [Google Scholar]
- Rappaport I. Some studies of the infectious process with MS2 bacteriophage. Biochim Biophys Acta. 1965 Jul 15;103(3):486–494. doi: 10.1016/0005-2787(65)90141-3. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Steitz J. E. The reconstitution of infective bacteriophage R17. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1416–1421. doi: 10.1073/pnas.58.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrmann G. F., Krueger R. G. Precipitation and neutralization of bacteriophage MS-2 by rabbit antibodies. J Immunol. 1970 Feb;104(2):353–358. [PubMed] [Google Scholar]
- Rohrmann G. F., Krueger R. G. The self-assembly of RNA free protein subunits from bacteriophage MS-2. Biochem Biophys Res Commun. 1970 Feb 6;38(3):406–413. doi: 10.1016/0006-291x(70)90728-x. [DOI] [PubMed] [Google Scholar]
- SCHACHMAN H. K., GROPPER L., HANLON S., PUTNEY F. Ultracentrifuge studies with absorption optics. II. Incorporation of a monochromator and its application to the study of proteins and interacting systems. Arch Biochem Biophys. 1962 Oct;99:175–190. doi: 10.1016/0003-9861(62)90259-x. [DOI] [PubMed] [Google Scholar]
- SCHARFF M. D., MAIZEL J. V., Jr, LEVINTOW L. PHYSICAL AND IMMUNOLOGICAL PROPERTIES OF A SOLUBLE PRECURSOR OF THE POLIOVIRUS CAPSID. Proc Natl Acad Sci U S A. 1964 Feb;51:329–337. doi: 10.1073/pnas.51.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT D. W. SEROLOGICAL CROSS REACTIONS AMONG THE RNA-CONTAINING COLIPHAGES. Virology. 1965 May;26:85–88. doi: 10.1016/0042-6822(65)90028-0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Identification of the A protein as a structural component of bacteriophage R17. J Mol Biol. 1968 May 14;33(3):923–936. doi: 10.1016/0022-2836(68)90328-8. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Isolation of the A protein from bacteriphage R17. J Mol Biol. 1968 May 14;33(3):937–945. doi: 10.1016/0022-2836(68)90329-x. [DOI] [PubMed] [Google Scholar]
- Vasquez C., Granboulan N., Franklin R. M. Structure of the ribonucleic acid bacteriophage R17. J Bacteriol. 1966 Dec;92(6):1779–1786. doi: 10.1128/jb.92.6.1779-1786.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viñuela E., Algranati I. D., Ochoa S. Synthesis of virus-specific proteins in Escherichia coli infected with the RNA bacteriophage MS2. Eur J Biochem. 1967 Mar;1(1):3–11. doi: 10.1007/978-3-662-25813-2_2. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]