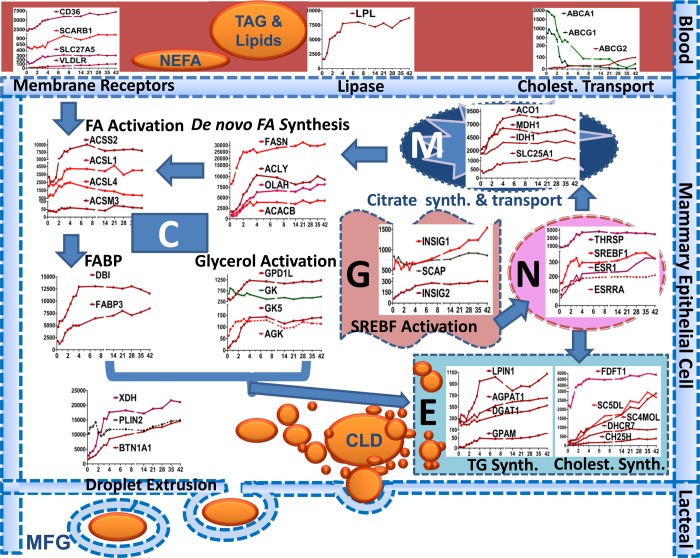
Fig. 8.
This figure depicts a model of the lipid synthesis genes in the human mammary epithelial cell during secretory activation according to cell compartment. C, cytoplasm; M, mitochondria; N, nucleus; E, endoplasmic reticulum; G, Golgi; CLD, cytoplasmic lipid droplet, MFG, milk fat globule. The VLDL-TG core (or chylomicrons) is hydrolyzed by LPL in cooperation with VLDLR and releases LCFA for transport across the endothelium into the extracellular space. LCFA are imported into mammary cells via CD36 in concert with SLC27A5. Once LCFAs cross the membrane, most are activated into an LC-acyl-CoA (LCACoA) primarily via ACSL1 & 4 for subsequent utilization. ACACA & B synthesize malonyl-CoA, and both acetyl-CoA and malonyl-CoA are substrates for FASN. Formation of acetyl-CoA from acetate is carried out by ACSS2. Acetyl CoA can also be derived from citrate by action of ACLY. Mitochondria generate ATP, protons, and citrate as fuel and substrate for de novo FA synthesis. OLAH (thioestrase II) interacts with FASN to produce medium-chain FA (C6-C14) and subsequent activation by ACSM3, which allows FAs to enter into the TG synthesis pathway. Glycerol and acylglycerols are activated by GK, GK5, and AGK. LCACoA bind primarily to DBI and FABP3, which transports them to specific intracellular organelles for serving as substrate for TG synthesis. Activated glycerol and LCACoA form TG via sequential reactions carried out by GPAM, AGPAT1, LIPIN1, and DGAT1. Cholesterol is mainly synthesized within the MEC (overall upregulation of genes responsible for synthesis) and little is imported (downregulation of transporters except ABCG2). Once TG and cholesterol are formed, they are inserted into the ER membrane to form tiny CLD. These fuse to form bigger CLD and are secreted, by an apocrine mechanism, surrounded by apical membrane into the alveolar lumen as MFG through the interaction of PLIN2 with BTN1A1 and XDH. The outer MFG membrane contains, among other membrane proteins derived from the apical membrane, lipid transporters and cholesterol. We propose that a role for SREBF1 and THSRP activation, via prolactin receptor signaling (STAT5B) is crucial in activation of genes involved in lipolysis, de novo synthesis, FA activation, and TG and cholesterol biosynthesis. SREBF1 is not active in the nucleus until cleaved by a process in the Golgi involving SCAP and INSIG. ESRRA activation via PPARA triggers upregulation of genes involved in FA transporter (CD36), lipase (LPL), FA binding (FABP3), and FA activation (ACSL1).