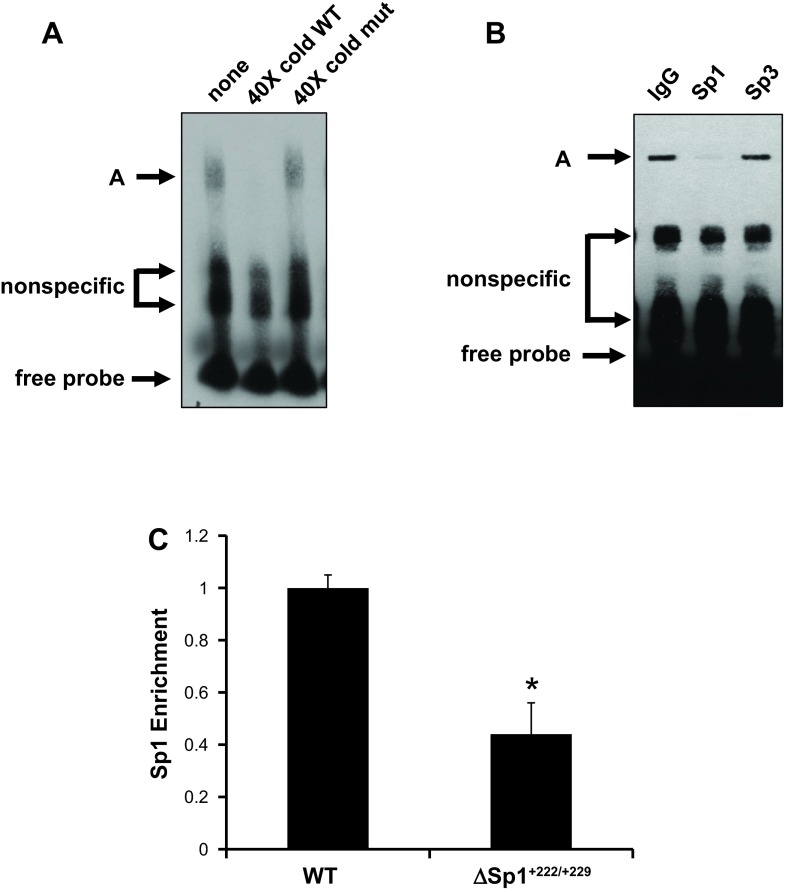
Fig. 2.
Sp1 protein binds to +222/+229 of the αENaC promoter. A: EMSA in which a biotin-labeled duplex probe corresponding to +208 to +240 of the mouse αENaC promoter was incubated with 10 μg of mouse inner medullary collecting duct (mIMCD)3 cell nuclear extracts alone (lane 1) or in the presence of a 40-fold molar excess of wild-type unlabeled probe (lane 2) or mutant unlabeled probe (lane 3). Gel is representative of 4 experiments. B: antibody interference assays were performed using 2 μg of nonimmune IgG or rabbit polyclonal antibodies against Sp1 or Sp3 (as a specificity control). Sp1 antibody decreased formation of complex A, whereas Sp3 antibody had no effect. Gel is representative of 4 experiments. C: mutations of the +222/+229 site as in Fig. 1 were introduced into pcDNA3.1-Zeo-1.3ENaCα-Luc to create pcDNA3.1-Zeo-1.3ENaCαΔSp1+222/+229-Luc as described under materials and methods. Stable mIMCD3 cell lines harboring the pcDNA3.1-Zeo-1.3ENaCα-Luc (WT) or pcDNA3.1-Zeo-1.3ENaCαΔSp1+222/+229-Luc (Δ+222/+229) constructs were established. Chromatin immunoprecipitation (ChIP)/qPCR experiments with anti-Sp1 antibody and primers to amplify the R3 subregion were conducted. The value for the final amplicon/input DNA obtained from the WT cells was set as 1, and that of the Δ+222/+229 was normalized to it. Error bars indicate ± SE. *P < 0.05 vs. WT; n = 3.