Abstract
The present renal hemodynamic study tested the hypothesis that CD38 and superoxide anion (O2·−) participate in the vasoconstriction produced by activation of thromboxane prostanoid (TP) receptors in the mouse kidney. CD38 is the major mammalian ADP-ribosyl cyclase contributing to vasomotor tone through the generation of cADP-ribose, a second messenger that activates ryanodine receptors to release Ca2+ from the sarcoplasmic reticulum in vascular smooth muscle cells. We evaluated whether the stable thromboxane mimetic U-46619 causes less pronounced renal vasoconstriction in CD38-deficient mice and the involvement of O2·− in U-46619-induced renal vasoconstriction. Our results indicate that U-46619 activation of TP receptors causes renal vasoconstriction in part by activating cADP-ribose signaling in renal resistance arterioles. Based on maximal renal blood flow and renal vascular resistance responses to bolus injections of U-46619, CD38 contributes 30–40% of the TP receptor-induced vasoconstriction. We also found that the antioxidant SOD mimetic tempol attenuated the magnitude of vasoconstriction by U-46619 in both groups of mice, suggesting mediation by O2·−. The degree of tempol blockage of U-46619-induced renal vasoconstriction was greater in wild-type mice, attenuating renal vasoconstriction by 40% compared with 30% in CD38-null mice. In other experiments, U-46619 rapidly stimulated O2·− production (dihydroethidium fluorescence) in isolated mouse afferent arterioles, an effect abolished by tempol. These observations provide the first in vivo demonstration of CD38 and O2·− involvement in the vasoconstrictor effects of TP receptor activation in the kidney and in vitro evidence for TP receptor stimulation of O2·− production by the afferent arteriole.
Keywords: kidney, renal circulation, renal vascular resistance, afferent arteriole, U-46619, thromboxane prostanoid receptor, oxidative stress, O2·− production, superoxide dismutase, tempol
the principal functions of vascular thromboxane [thromboxane A2 (TxA2)] prostanoid (TP) receptors are linked to vasoconstriction and hypertension as well as remodeling of vascular smooth muscle cells (VSMCs) and hemostasis (23, 50). Our interest in TP receptor function in the kidneys is based on its vasoconstrictor activity and its participation in the development of hypertension. Renal vasoconstriction is commonly associated with reduced Na+ excretion, a rightward shift in the pressure-natriuresis relationship, and increased arterial pressure (AP). In most models of hypertension, the kidneys undergo oxidative stress and excrete increased amounts of thromboxane and isoprostanes, both of which activate TP receptors and exacerbate renal damage and oxidative stress. Accordingly, TP receptors are critically involved in the chronic prohypertensive actions in various models such as angiotensin II (ANG II) infusion (11, 25, 27, 36, 40) and nitric oxide (NO) synthase inhibition (12, 47). Moreover, TP receptor activation contributes importantly to renal vasoconstriction in ANG II-induced hypertension (24, 36) and the nonclipped kidney in renovascular two-kidney, one-clip hypertension (18, 26).
Despite their functional importance, there is no consensus on the cellular signaling mechanisms mediating the vasomotor actions of TP receptors. These may differ according to particular tissues and vascular beds, perhaps with respect to vascular function (conduit vs. resistance artery/arteriole), possibly reflecting differential coupling of specific receptors to G protein families (41). In the aorta, TP receptor stimulation causes contraction by a combination of Ca2+ entry and mobilization from the sarcoplasmic reticulum (33). U-46619-induced constriction by coronary arteries is mediated by the entry of extracellular Ca2+ through dihydropyridine-sensitive voltage-gated L-type Ca2+ channels along with inositol 1,4,5-trisphosphate-mediated Ca2+ mobilization (53, 54), whereas Ca2+ mobilization takes place from ryanodine-sensitive stores in omental arteries (35).
The stable TxA2 mimetic U-46619 activates TP receptors in the renal microcirculation to reduce renal blood flow (RBF) and glomerular filtration rate as a result of a predominant increase in afferent arteriolar resistance in healthy animals (4). Some studies have shown that this renal vasoconstrictor action of U-46619 is almost completely blocked by the L-type channel blocker diltiazem (32) and afferent arterioles respond to inhibition of L-type Ca2+ channels with 80–85% inhibition of TP receptor-induced constriction in the chronically hydronephrotic rat kidney preparation (16). In contrast, a study (3) on isolated perfused rat kidneys reported that U-46619-induced vasoconstriction was relatively unresponsive to the L-type Ca2+ channel antagonist nifedipine but was markedly attenuated by Rho kinase inhibition. Thus, the relative roles of different modes of Ca2+ signaling and sensitization of the contractile apparatus produced by TP receptors in the renal microcirculation are uncertain.
Recently, it has become clear that part of the renal vasoconstriction resulting from activation of G protein-coupled receptors (GPCRs) such as ANG II type 1, adenosine A1, α-adrenoceptors, and endothelin (ET)A and ETB receptors depends on the generation of ROS, particularly superoxide (O2·−), and can be attenuated by the SOD mimetic tempol (15, 21, 31). The constrictor effect of O2·− can result from direct activation of excitation-contraction pathways in VSMCs (7), indirectly through inactivation of the vasodilator NO (20, 28, 31, 38), or a combination of both actions (21, 22, 30). Contributing to the vasoconstrictor effect of O2·− in the renal and coronary microcirculation is activation of the ectoenzyme CD38, the major mammalian ADP-ribosyl cyclase (1, 9, 10, 56). CD38 catalyzes the production of cADP-ribose from NAD+, which then acts as a second messenger to promote ryanodine-sensitive Ca2+ release from the sarcoplasmic reticulum of VSMCs (1, 55). CD38 is activated by O2·− in cardiac myocytes and isolated renal resistance vessels (9, 10, 56), providing an important link between vasoconstrictor GPCR activation and cADP-ribose generation by CD38. The importance of this pathway has been highlighted in pharmacological studies in the rat in which ADP-ribosyl cyclase activity was inhibited by nicotinamide and in mice genetically devoid of CD38. Both models have established that cADP-ribose production provides a significant contribution to the vasoconstrictor actions of ANG II, ET-1, and norepinephrine (NE) in the kidney (21, 22, 45).
Little is known about the importance of ADP-ribosyl cyclase in the vascular response to TP receptor stimulation. Although it has been reported to play an important role in the coronary circulation (13), it appears to be nonessential in isolated intrarenal arteries (44). In the present study, we tested the hypothesis that CD38 and O2·− participate in the vasoconstriction produced by TP receptors in the mouse kidney. We evaluated whether TP receptor activation by the stable TxA2 mimetic U-46619 causes renal vasoconstriction by stimulation of CD38 and downstream Ca2+ signaling such that less pronounced renal vasoconstriction was observed in CD38-deficient (CD28−/−) mice. We also assessed the involvement of O2·− in U-46619-induced renal vasoconstriction and found that the antioxidant SOD mimetic tempol attenuated the magnitude of vasoconstriction both wild-type (WT) and CD38−/− mice.
METHODS
C57-BL6j mice homozygous for deletion of the CD38 gene (CD38−/− mice) were bred in our facility from stock provided by Dr. Frances Lund (Trudeau University) along with C57BL6 WT controls (45). Animals were kept on a 12:12-h light-dark cycle with standard laboratory chow (Taconic TD99215, 0.4% NaCl) and tap water provided ad libitum. All procedures conformed with National Institutes of Health (NIH) guidelines for the care and use of laboratory animals. The experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
In Vivo Hemodynamic Experiments
Anesthesia was induced by an intraperitoneal injection of pentobarbital sodium (90 mg/kg, Sigma). Animals were placed on a servo-controlled heated table to maintain body temperature at 37°C (45). After installation of a tracheotomy tube [polyethylene (PE)-90] for airway maintenance, a jugular vein was cannulated with three pulled PE-10 cannulas. One cannula provided a continuous infusion of isotonic saline (0.9% NaCl) at 0.3 μl·min−1·g body wt−1, the second cannula provided 2% BSA solution (fraction V, Sigma) in isotonic saline at 3 μl/min, and the third cannula was used for supplemental doses of pentobarbital sodium as needed. AP was monitored through a PE-10 cannula inserted in a carotid artery using a strain-gauge transducer (model p23dB, Statham) and a Hewlett-Packard carrier amplifier (model 8805B). The urinary bladder was exposed through a suprapubic incision and cannulated at the dome with PE-50 tubing for urine drainage. The right kidney was exposed in the retroperitoneal space through a flank incision. The renal artery was cleared of connective tissue and positioned in a transit-time ultrasonic flow probe for measurement of RBF (Transonic 0.5PSB transducer connected to a TS420 flow monitor). The experimental kidney was denervated to isolate it from vasomotor input from the sympathetic nervous system. Animals were allowed to stabilize 30–45 min before data were recorded. Analog AP and RBF signals were digitized with a PC-based data-acquisition system (Data Translations DT9816 analog-to-digital board controlled by DtEz software) at 120 samples/s and averaged over 1-s intervals before being written to disk for offline analysis.
Renal Responses to TP Receptor Activation
Bolus intravenous injections of the TxA2 mimetic U-46619 were administered using a 10 μl HPLC injection valve connected in series with the BSA infusion line. For each injection, the infusion rate was increased to 60 μl/min immediately before the injection valve was operated and then returned to 3 μl/min when the injection had completed, 18 s later. This procedure provided consistent 10-μl bolus injections of U-46619 at a rate of 1 μl/s. U-46619 doses of 10, 25, and 50 ng were administered in random order (equivalent to 30, 75, and 150 pmol, respectively). All doses were administered in each period in duplicate. The recovery period after each injection was based on the time required for AP to return to the preinjection level, but was always at least 10 min. This procedure limits activation of the TP receptor to a minimum and minimizes tachyphylaxis associated with prolonged receptor activation.
Role of superoxide in TP receptor-mediated vasoconstriction.
Similar RBF experiments were conducted to investigate the role of superoxide in the vasoconstriction caused by TP receptor activation. Control responses to U-46619 were determined in a 60-min control period followed by a 60-min experimental period during which the SOD mimetic tempol was infused at 2 mg·min−1·kg−1. U-46619 responses in the experimental period were determined 20 min after the start of tempol administration.
Mediation of U-46619 responses by the TP receptor.
The specificity of the renal and systemic vascular responses to bolus injections of U-46619 was tested in a separate group of five mice that received an infusion of the selective TP receptor antagonist SQ-29,548 (3.3 μg·min−1·kg body wt−1) in place of tempol (39, 48). Additionally, a time-control series of five mice received saline in place of the tempol or SQ-29,548 infusion beginning at the end of the standard control period. Responses to U-46619 were recorded during the initial control period (0–60 min) and during a second control period (80–140 min). The protocol and recording times were similar to those for the second set of RBF experiments with the exception of tempol infusion.
Basal control values for RBF and renal vascular resistance (RVR) as well as mean AP (MAP) were averaged over 60 s immediately before each injection. Transient responses to U-46619 injections are presented as percent changes from the control value. Data were graphed and analyzed with a statistical software package (Prism 6.1, GraphPad). Renal hemodynamic responses were analyzed by linear regression of dose-response relationships over the U-46619 dose range. Group means were compared by paired or unpaired t-tests and one-way or two-way ANOVA as appropriate. P values of <0.05 were considered statistically significant.
Superoxide Activation by U-46619 in Isolated Afferent Arterioles
Afferent arterioles (<20 μm in diameter) were isolated from kidneys of WT mice (5–9 wk old, 19–27 g body wt) using procedures previously described by our laboratory (9, 10). Briefly, kidneys were infused via the abdominal aorta with ice-cold PBS [Ca2+-free PBS, containing (in mM) 138 NaCl, 2.67 KCl, 1.47 KH2PO4, and 8.1 Na2HPO4] until the effluent was clear. A suspension of magnetic polystyrene microspheres [1.5 ml of 0.8% (wt/vol), 4.2 μm, Spherotech, Libertyville, IL] was then infused, followed by kidney excision. Thin cortical slices were minced and homogenized before resuspension in 5 ml ice-cold PBS. The suspension was passed through a series of trituration and sieving steps to disperse the tissue and shear away tubules and connective tissue (9, 10). Enriched preparations of afferent arterioles containing magnetic beads were extracted with a magnet. Isolated arterioles with attached glomeruli were transferred to glass-bottom petri dishes (MatTek) precoated with Cell Tak (BD Biosciences).
The fluorescent dye dihydroethidium (DHE; Life Technologies) was used to evaluate superoxide production by individual afferent arterioles. A vessel was incubated for 30 min at room temperature in the dark in HBSS [composed of (in mM) 138 NaCl, 5.3 KCl, 0.34 Na2HPO4, 0.44 KH2PO4, 4.17 NaHCO3, 5.56 d-glucose] containing 2 μM DHE. After being washed with HBSS, vessels were kept in Ca2+-containing PBS (supplemented with 0.49 mM MgCl2 and 0.90 mM CaCl2) for 30 min a room temperature immediately before being imaged. Some arterioles were incubated with tempol (1 mM) in PBS for 30 min before the response to U-46619 was assessed (9, 10). Images were recorded at 5-s intervals for 325 s using a Zeiss LSM 510 inverted confocal microscope with 488-nm laser excitation and a long-pass 560-nm emission filter. After a 25-s control period, vessels were stimulated with U-46619 (2 μM) in PBS or PBS alone as a negative control. Fluorescent signals (mean grayscale intensity) were analyzed using ImageJ software (NIH). Fluorescent intensities were normalized to the mean value observed during the 25-s control period.
Reagents
U-46619 and SQ-29,548 were purchased from Cayman Chemical. DHE was purchased from Life Technologies. Other agents (e.g., tempol, albumin, and pentobarbital sodium) were purchased from Sigma.
RESULTS
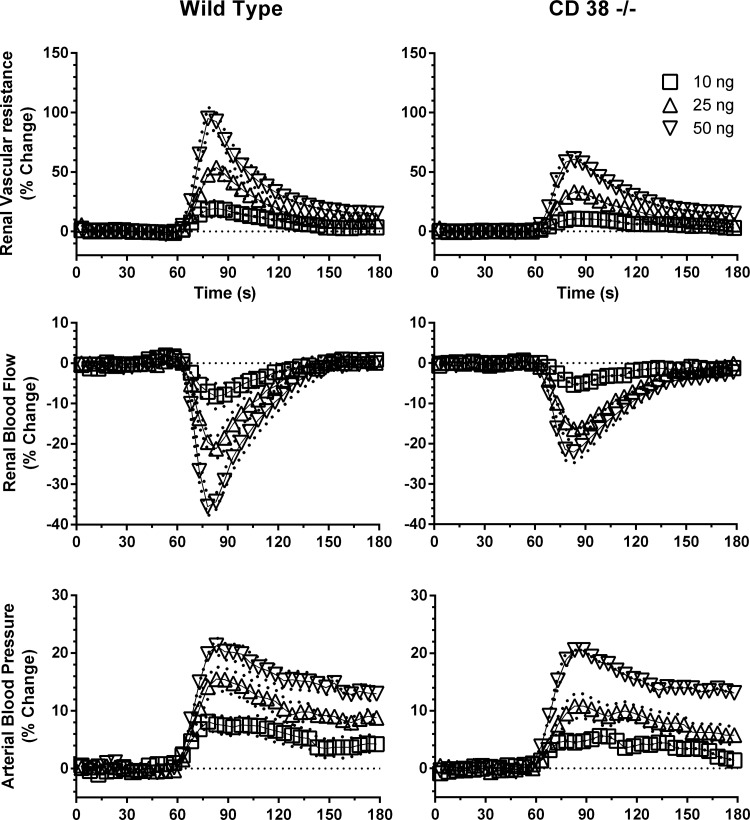
Control hemodynamic data for all animals used in the study are shown in Table 1. Under basal conditions, RBF, RVR, heart rate, and MAP were not different in WT and CD38−/− mice. Figure 1 shows group time-dependent responses to 10 μl iv bolus injections of U-46619 (10, 25, and 50 ng) administered at the 60-s time point in WT and CD38−/− mice. RBF rapidly declined and RVR and MAP increased in a dose-dependent manner. The renal hemodynamic response to U-46619 injection at 60 s was basically complete by 120 s. In contrast, the MAP pressor response took longer to recover to baseline, usually returning to the preinjection value by 5–8 min.
Table 1.
Basal renal hemodynamic data for all animals during control conditions
| Wild-Type Mice | CD 38−/− Mice | |
|---|---|---|
| Mean arterial pressure, mmHg | 96 ± 2 | 94 ± 3 |
| Renal blood flow, ml·min−1·g kidney wt−1 | 6.7 ± 0.4 | 7.8 ± 0.5 |
| Renal vascular resistance, mmHg·ml−1·min−1·g kidney wt | 15.4 ± 0.9 | 13.0 ± 0.7 |
| Heart rate, beats/min | 563 ± 29 | 559 ± 29 |
| Hematocrit, % | 45 ± 1 | 42 ± 2 |
| Right kidney weight, g | 0.18 ± 0.01 | 0.19 ± 0.01 |
| Body weight, g | 26 ± 2 | 26 ± 1 |
| Number of animals | 14 | 14 |
Values are means ± SE. There were no statistical differences between animal groups by an unpaired t-test.
Fig. 1.
Group mean time-dependent responses of renal blood flow (RBF), renal vascular resistance (RVR), and mean arterial pressure (MAP) to intravenous bolus injections of U-46619 (10, 25, and 50 ng) at 60 s in wild-type (WT) mice (n = 7) and CD38-deficient (CD38−/−) mice (n = 7). Values are means ± SE.
Renal Vasoconstriction by U46619 Is Attenuated in CD38−/− Mice
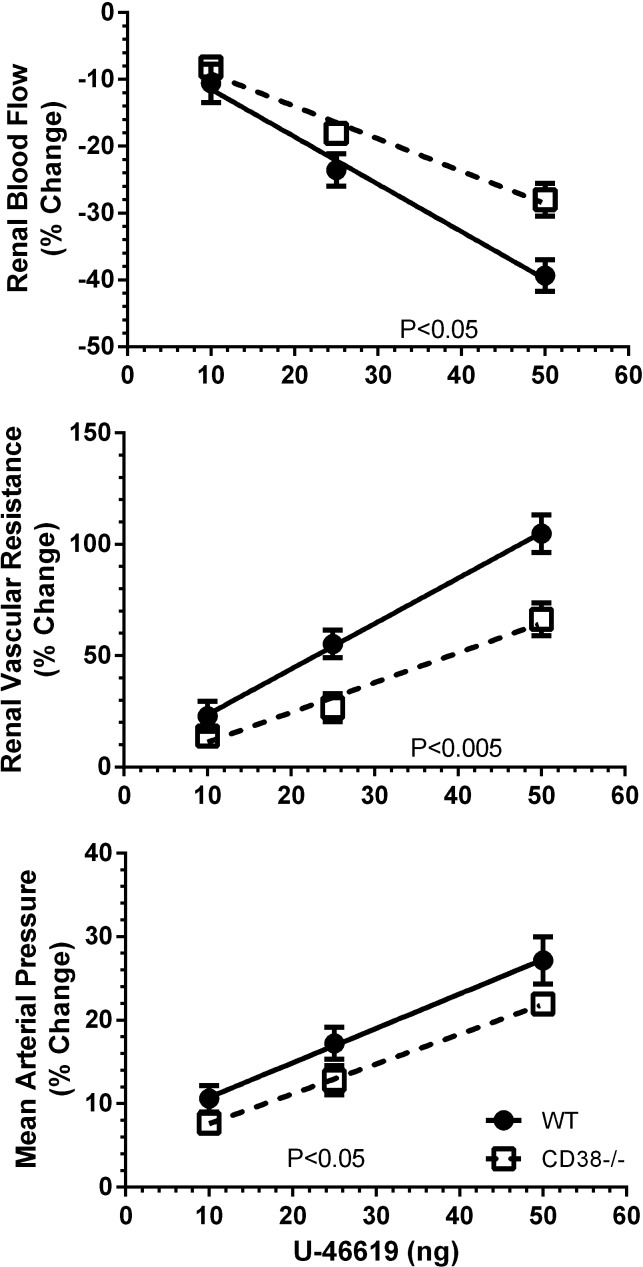
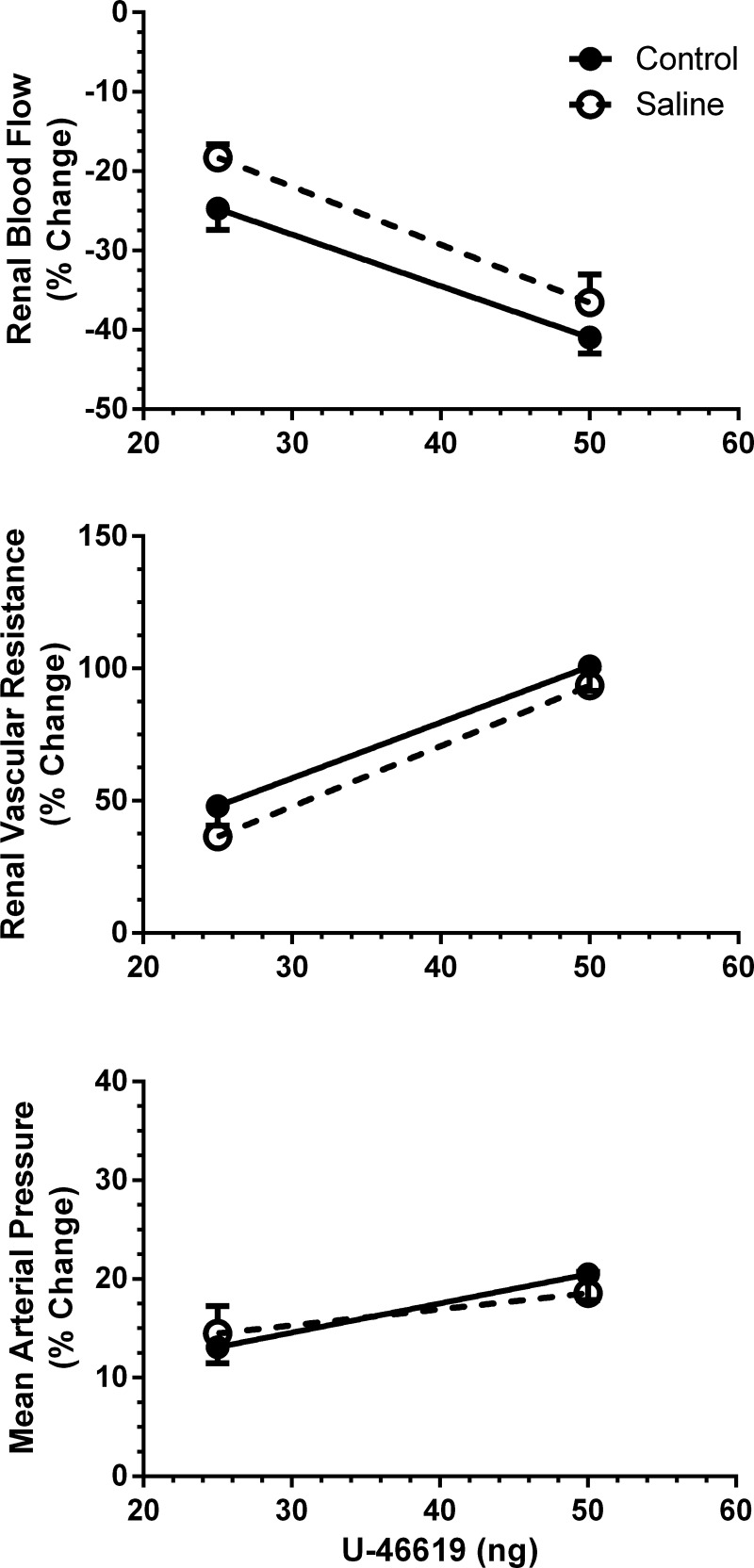
Figure 2 shows the maximum transient changes produced by U-46619. ANOVA of the overall data revealed dose-dependent effects of the TxA2 mimetic on RBF, RVR, and MAP in both groups of mice (P < 0.001 for all). This was the case for the changes expressed as percent changes from baseline and for absolute changes (data not shown). An important finding was that renal vasoconstriction was attenuated in CD38−/− animals. There were less pronounced changes in RBF and RVR for a given concentration of U-46619 in the absence of CD38. There was a similar tendency for MAP, but it did not attain statistical significance. These conclusions about strain differences are more stringently supported by analysis of the slopes of the dose-response relations, with weaker responses on the average for renal vasoconstriction and the pressor response to U-46619 in CD38−/− mice (P < 0.001; Table 2).
Fig. 2.
Maximum changes in RBF, RVR, and MAP to injections of U-46619 in WT mice (n = 7) and CD38−/− mice (n = 7). Slopes for the relations are shown in Table 2.
Table 2.
Slopes (percent changes from control or baseline/ng) of dose-response relations for the administration of U-46619 in wild-type and CD38−/− mice
| Wild-Type Mice | CD38−/− Mice | P Value | |
|---|---|---|---|
| Mean arterial pressure | 0.44 ± 0.03 | 0.37 ± 0.01 | <0.001 |
| Renal blood flow | −0.76 ± 0.06 | −0.51 ± 0.05 | <0.001 |
| Renal vascular resistance | 2.11 ± 0.08 | 1.29 ± 0.12 | <0.001 |
Values are means ± SE. Slope comparisons were by an extra sum of squares F-test.
The possible role of ROS and O2·− was evaluated in a second group of animals by comparing renal and systemic hemodynamic responses to U-46619 injections before and during intravenous infusion of the SOD mimetic tempol. Basal renal hemodynamic values during control and tempol infusion periods before U-46619 administration are shown in Table 3. There were no group differences in RBF, RVR, or MAP between WT and CD38−/− mice during both control and tempol infusion periods. Tempol reduced MAP by 12–15 mmHg and reduced RVR by ∼20% in both groups of mice (P < 0.05), whereas RBF tended to increase slightly by 5–10% during tempol administration (P = not significant).
Table 3.
Basal renal hemodynamic data for wild-type and CD38−/− mice before and after intravenous infusion of tempol
| Wild-Type Mice |
CD38−/− Mice |
|||
|---|---|---|---|---|
| Control | Tempol | Control | Tempol | |
| Mean arterial pressure, mmHg | 94 ± 2 | 79 ± 3** | 99 ± 5 | 87 ± 6* |
| Renal blood flow, ml·min−1·g kidney wt−1 | 6.3 ± 0.7 | 6.6 ± 0.8 | 8.0 ± 0.8 | 8.8 ± 0.9 |
| Renal vascular resistance, mmHg·ml−1·min−1·g kidney wt | 16.2 ± 1.4 | 12.9 ± 1.5* | 13.6 ± 1.8 | 10.9 ± 1.4** |
| Heart rate, beats/min | 561 ± 40 | 570 ± 30 | 572 ± 17 | 609 ± 34 |
| Hematocrit, % | 42 ± 2 | 44 ± 1 | ||
| Right kidney weight, g | 0.17 ± 0.01 | 0.17 ± 0.02 | ||
| Number of animals | 7 | 7 | ||
P < 0.05 for comparison between WT and CD38−/− mice in the same control or tempol period;
P < 0.01 for paired comparison between control and tempol periods in the same group. There were no significant differences between stains in either control or tempol periods (by ANOVA).
Tempol Inhibits U-46619-Mediated Renal Vasoconstriction
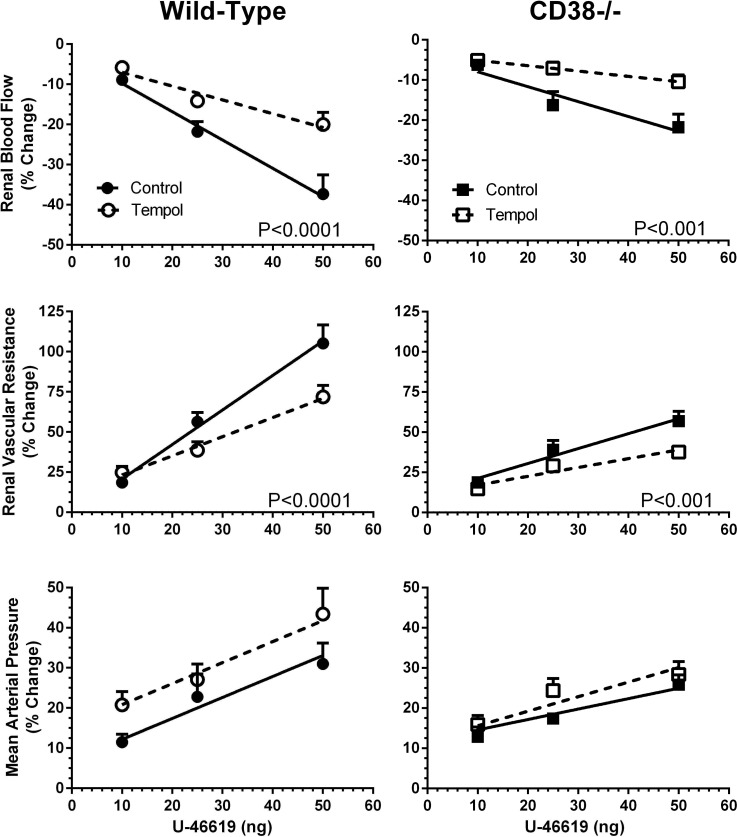
The effects of tempol scavenging of O2·− on maximum cardiorenal responses, expressed as percent changes from control or baseline, to U-46619 are shown in Fig. 3. RBF and RVR responses to U-46619 were attenuated by the antioxidant tempol (P < 0.001). This was the case in both WT and CD38−/− mice. Thus, tempol scavenging of O2·− reduced U-46619-induced renal vasoconstriction. Tempol attenuated RBF responses to 25- and 50-ng doses of U-46619 by ∼50%. TP receptor-mediated increases in RVR were reduced by tempol by ∼35% in both groups. In contrast to the antioxidant effects in the kidney, the systemic vasoconstrictor response to U-46619 based on MAP was enhanced somewhat in WT mice during tempol infusion. However, the slope for the dose-response effect of U-46619 on MAP was unaffected by tempol in both groups of animals (Fig. 3). Thus, the MAP response to the TxA2 mimetic did not differ appreciably between mouse groups either before or during tempol infusion.
Fig. 3.
Maximum changes in RBF, RVR, and MAP to U-46619 administration during control and experimental (tempol) periods in WT mice (n = 7) and CD38−/− mice (n = 7). Values are means ± SE.
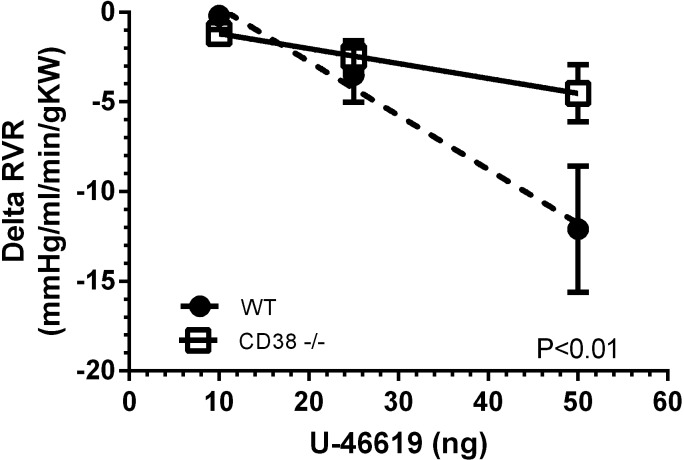
Figure 4 shows a small strain difference in the effect of tempol on the maximum renal vascular responses (expressed in absolute units) to U-46619. ANOVA revealed that tempol scavenging of O2·− reduced the absolute change in RVR to U-46619 more in WT mice than in CD38−/− mice (P < 0.05). This observation is consistent with the notion that O2·− increases RVR during TP receptor stimulation in part by activation of CD38. Similar analyses of absolute changes in RBF or MAP did not reveal a statistically significant strain difference in the effect of tempol.
Fig. 4.
Effect of the SOD mimetic tempol on the response of RVR (expressed in absolute units) to U-46619 in WT mice (n = 7) and CD38−/− mice (n = 7). The tempol effect is defined as the difference of U-46619 responses between tempol and control periods.
TP Receptors Mediate Renal Hemodynamic Responses to U-46619
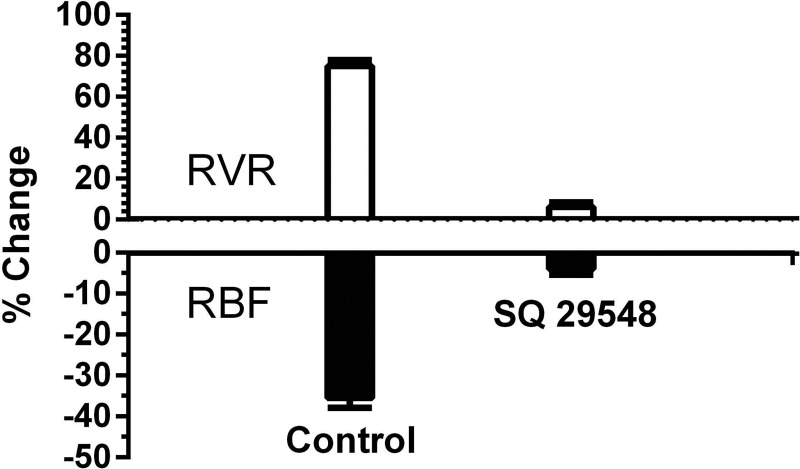
The effect of TP receptor inhibition with SQ-29,548 on renal vascular responses to U-46619 is shown in Fig. 5. The 36% maximum increase in RVR caused by U-46619 (50 ng bolus) was reduced to 6% during inhibition of TP receptors with SQ-29,548 (P < 0.001). Similarly, the maximum 35% reduction in RBF was reduced to 4% by TP receptor antagonism (P < 0.001). These results demonstrate the electivity of U-46619 for TP receptors in the renal vascular responses we observed.
Fig. 5.
Inhibition of U-46619-mediated renal vasoconstriction with the selective thromboxane prostanoid receptor antagonist SQ-29,548. Responses of RBF and RVR to U-46619 (50 ng bolus iv) before (control) and during infusion of SQ-29,548 in WT mice (n = 5) are shown.
The stability of the preparation and consistency of renal hemodynamic responses to U-46619 are shown in Fig. 6, which shows data from a time-control group of WT mice. Saline was infused throughout the experiment in control period 1 (0–60 min) and control period 2 (80–140 min); the latter corresponded with the experimental period when tempol was used in the earlier experiments. Data analysis via ANOVA revealed no time-dependent changes in RBF, RVR, or MAP responses to U-46619 (50 ng).
Fig. 6.
Time-control series showing the uniformity of U-46619 effects on RBF, RVR, and MAP as a function of time in control period 1 (0–60 min) and control period 2 (80–140 min) in WT mice (n = 6).
U-46619 Stimulates Superoxide Production by the Afferent Arteriole
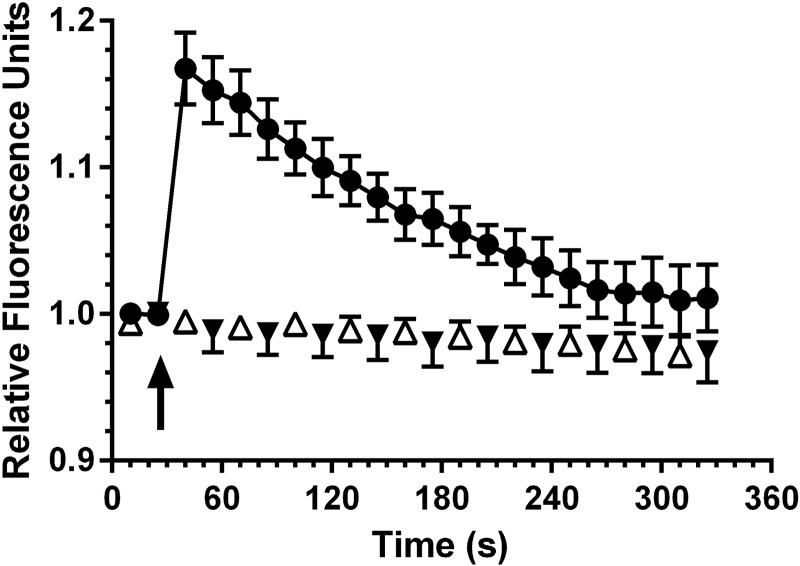
O2·− production measured by DHE fluorescence before and after U-46619 stimulation of isolated afferent arterioles is shown in Fig. 7. After a 25-s control period, addition of U-46619 caused an abrupt increase in DHE fluorescence, which peaked at 18% above baseline at 30 s and then declined progressively toward prestimulation levels over the subsequent 2.5 min. Also presented are time-control data showing the response to PBS addition rather than the TxA2 mimetic. An important observation was that prior exposure of arterioles to tempol completely eliminated the O2·− fluorescence response to U-46619. The average recording did not differ from fluorescence noted in the PBS time-control group. Fluorescence intensity actually declined by 6% over the 325-s recording in time-control and tempol-treated vessels. These results demonstrate that the measured response is likely the result of superoxide generation in response to TP receptor activation and not H2O2.
Fig. 7.
Acute effect of U-46619 (2 μM) on superoxide production as indicated by dihydroethidium fluorescence in isolated afferent arterioles from WT mice (●; n = 6). U-46619 was added at 30 s. Preexposure of arterioles to tempol (▼; n = 6) eliminated the response to U-46619, which was indistinguishable from the time-control series given PBS vehicle rather than the thromboxane A2 mimetic (△; n = 6).
DISCUSSION
The purpose of our study was to evaluate the extent to which that the major mammalian ADP-ribosyl cyclase CD38 and superoxide participate in the acute vasoconstriction produced by TP receptors in the mouse kidney. We tested the hypothesis that TP receptor activation by the stable thromboxane mimetic U-46619 causes renal vasoconstriction by stimulation of CD38 and downstream Ca2+ signaling such that renal vasoconstriction is less pronounced in CD38−/− mice. We also evaluated the involvement of ROS in U-46619-induced renal vasoconstriction by determining the efficacy of the antioxidant SOD mimetic tempol in attenuating the magnitude of vasoconstriction.
One of our new findings is that the ADP-ribosyl cyclase CD38 and cADP-ribose signaling are responsible in large part for TP receptor-mediated renal vasoconstriction. Based on in vivo maximal RBF and RVR responses to bolus injections of U-46619 in WT and CD38−/− mice, CD38 contributes ∼30–40% of the TP receptor-induced vasoconstriction. The reason why an earlier study (44) of isolated relatively large intrarenal arteries did not find evidence for significant ADP-ribosyl cyclase signaling is not known.
As previously reported, basal renal hemodynamics or MAP do not differ markedly between CD38−/− and WT mice (45). Our earlier RBF study (45) demonstrated that vasoconstrictor agents such as ANG II, ET-1, and NE cause less pronounced increases in RVR in CD38−/− versus WT mice. Consistent with this observation, pharmacological inhibition of ADP-ribosyl cyclase and ryanodine receptor (RyR) activity contribute to basal renal vasomotor tone as well as agonist-induced renal vasoconstriction in the rat (46). The ADP-ribosyl cyclase/RyR component of renal vasoconstriction is related to increased cytosolic Ca2+ concentration ([Ca2+]i), with cADP-ribose acting as a second messenger to mobilize intracellular Ca2+ independent of inositol 1,4,5-trisphosphate (1). This is observed in renal afferent arterioles and their individual VSMCs (8–10). These same GPCR agonists promptly stimulate vascular NADPH oxidase to produce O2·−, a triggering event thought to enhance ADP-ribosyl cyclase activity (9, 10). Whether or not ANG II and ET-1 increase [Ca2+]i via ADP-ribosyl cyclase/cADP-ribose and RyR-induced Ca2+ release in VSMCs of other major nonrenal vascular beds, either in vitro or in vivo, is not clear. In one study (14), ET-1 was reported to activate this pathway in mesenteric arteries, whereas ET-1 actions on pulmonary arterial VSMCs appeared to be independent of cADP-ribose in another study (57). Muscarinic receptors activate cADP-ribose signal transduction in coronary arteries (55).
A second goal of our study was to determine the involvement of O2·− in the acute renal hemodynamic response to TP receptor activation. To this end, we used the membrane-permeable SOD mimetic tempol, an antioxidant that reduces O2·− levels in the vasculature and kidney (6, 29). Our results show that tempol attenuated U-46619-induced renal vasoconstriction in both WT and CD38−/− mice, indicating a strong vasoconstrictor action of O2·−. The degree of tempol blockage of U-46619-induced renal vasoconstriction was somewhat more pronounced in WT mice than in CD38 mutant mice, implying a greater magnitude of constriction produced by O2·− and an action of O2·− dependent in part on CD38. The antioxidant attenuated renal vasoconstriction by U-46619 by 40%, on average, in WT mice and 30% in CD38-null mice. Thus, O2·− appeared to stimulate CD38 to produce greater renal vasoconstriction in WT animals than in CD38−/− animals. A relatively small component of O2·− stimulation of CD38 was suggested by the greater action of tempol in scavenging O2·− on RVR changes (expressed in absolute units) in WT animals compared with CD38−/− animals. Nevertheless, the majority of O2·−-mediated renal vasoconstriction was independent of CD38. The extent to which this reflected O2·− quenching of NO compared with a direct action on VSMCs was not evaluated in the present study.
Previous studies (6, 21, 22, 34) have reported that tempol reduced RVR and vasoconstrictor responses to GPCR agonists such as ANG II, ET-1, and NE. To our knowledge, the possible involvement of ROS in vasoconstriction produced by TxA2 or isoprostane has not been tested in an animal before. In this regard, our tempol results indicated that O2·− contributes importantly to the renal vasoconstriction elicited by the TxA2 mimetic in both WT and CD38−/− mice. In addition, we present new information showing that U-4619 promptly increases O2·− production by afferent arterioles isolated from WT mice, an effect abolished by tempol. While tempol may have actions primarily as an antioxidant that scavenges O2·−, it may reduce sympathetic nerve activity (19). In this regard, it should be recognized that our mouse kidneys were denervated. Thus, changes in renal sympathetic nerve activity could not have contributed directly to the renal hemodynamic responses we observed. However, it is noteworthy that we observed some differences in comparing renal versus apparent systemic vasoconstriction based on changes in RBF and RBF versus those in MAP. Unlike the renal vasculature, which showed reduced vasoconstrictor responses to U-46619 after tempol, it is not clear why tempol did not reduce MAP responses to U-46619. Heart rate was unaffected by the SOD mimetic in our animals, and the increased pressor response seems to reflect a difference between renal and nonrenal vascular effects of tempol. A more comprehensive analysis would require determinations of regional vascular resistance in multiple vascular beds. In animals with ANG II-induced hypertension, tempol reduced MAP by 30% and total peripheral resistance by 35% without affecting local blood flow to the kidney, heart, mesentery, skeletal muscle, and lung, suggesting rather homogeneous responses among different vascular beds (38).
Our data on isolated afferent arterioles of WT mice demonstrated that TP receptor activation by U-46619 causes a prompt increase in superoxide generation. The inhibitory action of tempol implicates superoxide as the major ROS rather than H2O2. The inhibitory action of tempol on the renal vasoconstrictor action of U-46619 in vivo demonstrated the renal vascular consequences of this component of TP receptor activity. However, the mechanism behind this ROS-mediated vasoconstriction is not evident in these experiments. A previous study (43) reported that TP receptor-mediated vasoconstriction of microperfused rabbit afferent arterioles is modulated by opposing actions of O2·− and NO, with enhancement by inhibition of NO synthesis and attenuation using the SOD mimetic tempol. It is noteworthy that the antioxidant tempol completely abolished U-46619-induced vasoconstriction in that in vitro study but attenuated only ∼50% of the whole kidney vasoconstriction in vivo in our study. This may reflect incomplete destruction of O2·− or perhaps the in vivo contribution of other vessels in the renal microcirculation. On the other hand, TP receptor activation was able to cause constriction of isolated arterioles during NO synthase inhibition and O2·− scavenging. Enhanced contractility of the afferent arteriole to ANG II, ET-1, and U-46691 in ANG II-infused hypertensive rabbits involves interactions of endothelium-derived TxA2, TP receptors, and O2·− production (49). Exaggerated reactivity to ANG II, ET-1, and U-46619 was normalized by antagonism of TP receptors in combination with the SOD mimetic tempol. Our RBF experiments indicated that stimulation of cADP-ribose/RyR/Ca2+ mobilization contributes to the U-46619-induced renal vasoconstriction during tempol administration with a contribution from other signaling pathways. This is consistent with a report (3) showing that in addition to increasing [Ca2+]i, TP receptor activation can produce vasoconstriction by increasing Ca2+ sensitivity of the contractile apparatus in VSMCs by stimulation of Rho/Rho kinase. Furthermore, TP receptor activation of tubuloglomerular feedback represents a vasoconstrictor mechanism unique to the kidneys (5, 51).
The TP receptor and its agonists have complex interactions with ROS that are important in the development of renal diseases. In vitro studies have indicate that TxA2 activates NADPH oxidase and that TxA2 production is upregulated in cardiovascular and renal disease. TP receptor activation by U-46619 and 8-isoprostane F2α stimulates Rac-1-mediated NADPH oxidase 1, but not NADPH oxidase 4, expression and O2·− production in nonrenal VSMCs (37). In turn, ROS generation contracts aortic VSMCs via quenching of NO and by generating TxA2 and isoprostane, which activate TP receptors (17) to vasoconstrict independent of the presence of NO (42). Oxidative stress can regulate TP receptor signal transduction at multiple sites, including TP receptor stabilization in the plasma membrane and reduced internalization and degradation in VSMCs (2, 52). Thus, TxA2 both activates NADPH oxidase and is itself upregulated in cardiovascular and renal diseases, resulting in a self-amplifying cascade between TxA2 and TP receptors involving ROS generation.
Perspectives
Renal vasoconstriction is often associated with increased oxidative stress and high levels of isoprostanes. In pathophysiological conditions, exaggerated TP receptor activation likely contributes to renal vasoconstriction and prohypertensive actions. Our experiments provide new insights into the mechanisms by which TP receptors constrict the renal microcirculation. TP receptors stimulate CD38 and O2·−, each of which contributes to vasoconstriction. In addition, there appears to be some interaction in that O2·− stimulates CD38 to cause part of its action in addition to independent effects on RVR. Assignment of relative importance of these signaling pathways in different models of cardiovascular and renal disease awaits further investigation.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Research Grant HL-02334.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.G.M. and W.J.A. conception and design of research; N.G.M., P.A.V., and T.E.K. performed experiments; N.G.M., P.A.V., and W.J.A. analyzed data; N.G.M., P.A.V., and W.J.A. interpreted results of experiments; N.G.M. prepared figures; N.G.M., P.A.V., and W.J.A. edited and revised manuscript; N.G.M., P.A.V., T.E.K., and W.J.A. approved final version of manuscript; W.J.A. drafted manuscript.
REFERENCES
- 1.Arendshorst WJ, Thai TL. Regulation of the renal microcirculation by ryanodine receptors and calcium-induced calcium release. Cur Opin Nephrol Hypertens 18: 40–49, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Ball SK, Field MC, Tippins JR. Regulation of thromboxane receptor signaling at multiple levels by oxidative stress-induced stabilization, relocation and enhanced responsiveness. PLos One 5: e12798, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer J, Parekh N. Variations in cell signaling pathways for different vasoconstrictor agonists in renal circulation of the rat. Kidney Int 63: 2178–2186, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Baylis C. Effects of administered thromboxanes on the intact, normal rat kidney. Renal Physiol 10: 110–121, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Brannstrom K, Arendshorst WJ. Thromboxane A2 contributes to the enhanced tubuloglomerular feedback activity in young SHR. Am J Physiol Renal Physiol 276: F758–F766, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Chen YF, Cowley AW, Jr, Zou AP. Increased H2O2 counteracts the vasodilator and natriuretic effects of superoxide dismutation by tempol in renal medulla. Am J Physiol Regul Integr Comp Physiol 285: R827–R833, 2003 [DOI] [PubMed] [Google Scholar]
- 7.de Richeliew TT, Sorensen CM, Holstein-Rathlou NH, Salomonsson M. NO independent mechanism mediates tempol induced renal vasodilation in SHR. Am J Physiol Renal Physiol 289: F1227–F1234, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Fellner SK, Arendshorst WJ. Angiotensin II Ca2+ signaling in rat afferent arterioles: stimulation of cyclic ADP ribose and IP3 pathways. Am J Physiol Renal Physiol 288: F785–F791, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Fellner SK, Arendshorst WJ. Angiotensin II, reactive oxygen species, and Ca2+ signaling in afferent arterioles. Am J Physiol Renal Physiol 289: F1012–F1019, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Fellner SK, Arendshorst WJ. Endothelin-A and -B receptors, superoxide, and Ca2+ signaling in afferent arterioles. Am J Physiol Renal Physiol 292: F175–F184, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Francois H, Athirakul K, Mao L, Rockman H, Coffman TM. Role for thromboxane receptors in angiotensin-II-induced hypertension. Hypertension 43: 364–369, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Francois H, Makhanova N, Ruiz P, Ellison J, Mao L, Rockman HA, Coffman TM. A role for the thromboxane receptor in l-NAME hypertension. Am J Physiol Renal Physiol 295: F1096–F1102, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geiger J, Zou AP, Campbell WB, Li PL. Inhibition of cADP-ribose formation produces vasodilation in bovine coronary arteries. Hypertension 35: 397–402, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Giulumian AD, Meszaros LG, Fuchs LC. Endothelin-1-induced contraction of mesenteric small arteries is mediated by ryanodine receptor Ca2+ channels and cyclic ADP-ribose. J Cardiovasc Pharmacol 36: 758–763, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Haque MZ, Majid DSA. Assessment of renal functional phenotype in mice lacking gp91phox subunit of NAD(P)H oxidase. Hypertension 43: 335–340, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Hayashi K, Loutzenhiser RD, Epstein M. Direct evidence that thromboxane mimetic U44069 preferentially constricts the afferent arteriole. J Am Soc Neprol 8: 25–31, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Hibino M, Okumura K, Iwama Y, Mokuno S, Osanai H, Matsui H, Toki Y, Ito T. Oxygen-derived free radical-induced vasoconstriction by thromboxane A2 in aorta of the spontaneously hypertensive rat. J Cardiovasc Pharmacol 33: 605–610, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Himmelstein SI, Klotman PE. The role of thromboxane in two-kidney, one-clip Goldblatt hypertension in rats. Am J Physiol Renal Fluid Electrolyte Physiol 257: F190–F196, 1989 [DOI] [PubMed] [Google Scholar]
- 19.Hu L, Zhang Y, Lim PS, Miao Y, Tan C, McKenzie KU, Schyvens CG, Whitworth JA. Apocynin but not l-arginine prevents and reverses dexamethasone-induced hypertension in the rat. Am J Hypertens 19: 413–418, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Ichihara A, Hayashi M, Hirota N, Saruta T. Superoxide inhibits neuronal nitric oxide synthase influences on afferent arterioles in spontaneously hypertensive rats. Hypertension 37: 630–634, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Just A, Olson AJ, Whitten CL, Arendshorst WJ. Superoxide mediates acute renal vasoconstriction produced by angiotensin II and catecholamines by a mechanism independent of nitric oxide. Am J Physiol Heart Circ Physiol 292: H83–H92, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Just A, Whitten CL, Arendshorst WJ. Reactive oxygen species participate in acute renal vasoconstrictor responses induced by ETA- and ETB-receptors. Am J Physiol Renal Physiol 294: F719–F728, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Katz AM. Protein families that mediate Ca2+ signaling in the cardiovascular system. Am J Cardiol 78: 2–6, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Kaushal RD, Wilson TW. Effect of furegrelate on renal plasma flow after angiotensin II infusion. Can J Physiol Pharmacol 68: 500–504, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Kawada N, Dennehy K, Solis G, Modlinger P, Hamel R, Kawada JT, Aslam S, Moriyama T, Imai E, Welch WJ, Wilcox CS. TP receptors regulate renal hemodynamics during angiotensin II slow pressor response. Am J Physiol Renal Physiol 287: F753–F759, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Keen HL, Brands MW, Smith MJ, Jr, Shek EW, Hall JE. Inhibition of thromboxane synthesis attenuates insulin hypertension in rats. Am J Hypertens 10: 1125–1131, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Keen HL, Brands MW, Smith MJ, Jr, Shek EW, Hall JE. Thromboxane is required for full expression of angiotensin hypertension in rats. Hypertension 29: 310–314, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Kopkan L, Huskova Z, Vanourkova Z, Thumova M, Skaroupkova P, Cervenka L, Majid DSA. Superoxide and its interaction with nitric oxide modulates renal function in prehypertensive Ren-2 transgenic rats. J Hypertens 25: 2257–2265, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Lai EY, Wellstein A, Welch WJ, Wilcox CS. Superoxide modulates myogenic contractions of mouse afferent arterioles. Hypertension 58: 650–656, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu R, Ren Y, Garvin JL, Carretero OA. Superoxide enhances tubuloglomerular feedback by constricting the afferent arteriole. Kidney Int 66: 268–274, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Lopez B, Salom MG, Arregui B, Valero F, Fenoy FJ. Role of superoxide in modulating the renal effects of angiotensin II. Hypertension 42: 1150–1156, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Loutzenhiser RD, Epstein M, Horton C, Sonke P. Reversal of renal and smooth muscle actions of the thromboxane mimetic U-44069 by diltiazem. Am J Physiol Renal Fluid Electrolyte Physiol 250: F619–F626, 1986 [DOI] [PubMed] [Google Scholar]
- 33.Loutzenhiser RD, Van Breemen C. Mechanism of activation of isolated rabbit aorta by PGH2 analogue U-44069. Am J Physiol Cell Physiol 241: C243–C249, 1981 [DOI] [PubMed] [Google Scholar]
- 34.Majid DSA, Nishiyama A, Jackson KE, Castillo A. Superoxide scavenging attenuates renal responses to ANG II during nitric oxide synthase inhibition in anesthetized dogs. Am J Physiol Renal Physiol 288: F412–F419, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Martinez MC, Randriamboavonjy V, Ohlmann P, Komas N, Duarte J, Schneider F, Stoclet JC, Andriantsitohaina R. Involvement of protein kinase C, tyrosine kinases, and Rho kinase in Ca2+ handling of human small arteries. Am J Physiol Heart Circ Physiol 279: H1228–H1238, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Mistry M, Muirhead EE, Yamaguchi Y, Nasjletti A. Renal function in rats with angiotensin II-salt-induced hypertension: effect of thromboxane synthesis inhibition and receptor blockade. J Hypertens 8: 75–83, 1990 [DOI] [PubMed] [Google Scholar]
- 37.Muzaffar S, Shukla N, Bond M, Sala-Newby G, Angelini GD, Newby AC, Jeremy JY. Acute inhibition of superoxide formation and Rac1 activation by nitric oxide and iloprost in human vascular smooth muscle cells in response to the thromboxane A2 analogue, U46619. Prostaglandins Leukot Essent Fatty Acids 78: 247–255, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishiyama A, Fukui T, Fujisawa Y, Rahman M, Tian RX, Kimura S, Abe Y. Systemic and regional hemodynamic responses to tempol in angiotensin II-infused hypertensive rats. Hypertension 37: 77–83, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Ogletree ML, Harris DN, Greenberg R, Haslanger MF, Nakane M. Pharmacological actions of SQ 29,548, a novel selective thromboxane antagonist. J Pharmacol Exp Ther 234: 435–441, 1985 [PubMed] [Google Scholar]
- 40.Ortiz MC, Sanabria E, Manriquez MC, Romero JC, Juncos LA. Role of endothelin and isoprostanes in slow pressor responses to angiotensin II. Hypertension 37: 505–510, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Perez-Vizcaino F, Villamor E, Duarte J, Tamargo J. Involvement of protein kinase C in reduced relaxant responses to the NO/cyclic GMP pathway in piglet pulmonary arteries contracted by the thromboxane A2-mimetic U46619. Br J Pharmacol 121: 1323–1333, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfister SL, Nithipatikom K, Campbell WB. Role of superoxide and thromboxane receptors in acute angiotensin II-induced vasoconstriction of rabbit vessels. Am J Physiol Heart Circ Physiol 300: H2064–H2071, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schnackenberg CG, Welch WJ, Wilcox CS. TP receptor-mediated vasoconstriction in microperfused afferent arterioles: roles of O2− and NO. Am J Physiol Renal Physiol 279: F302–F308, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Teggatz EG, Zhang G, Zhang AY, Yi F, Li N, Zou AP, Li PL. Role of cyclic ADP-ribose in Ca2+-induced Ca2+ release and vasoconstriction in small renal arteries. Microvasc Res 70: 65–75, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Thai TL, Arendshorst WJ. Mice lacking the ADP ribosyl cyclase CD38 exhibit attenauated renal vasoconstriction to angiotensin II, endothelin-1, and norepinephrine. Am J Physiol Renal Physiol 297: F169–F176, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thai TL, Fellner SK, Arendshorst WJ. ADP-ribosyl cyclase and ryanodine receptor activity contribute to basal renal vasomotor tone and agonist-induced renal vasoconstriction in vivo. Am J Physiol Renal Physiol 293: F1107–F1114, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Tomida T, Numaguchi Y, Nishimoto Y, Tsuzuki M, Hayashi Y, Imai H, Matsui H, Okumura K. Inhibition of COX-2 prevents hypertension and proteinuria associated with a decrease of 8-iso-PGF2α formation in l-NAME-treated rats. J Hypertens 21: 601–609, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Vagnes OB, Iversen BM, Arendshorst WJ. Short-term ANG II produces renal vasoconstriction independent of TP receptor activation and TxA2/isoprostane production. Am J Physiol Renal Physiol 293: F860–F867, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Wang D, Chabrashvili T, Wilcox CS. Enhanced contractility of renal afferent arterioles from angiotensin-infused rabbits: roles of oxidative stress, thromboxane prostanoid receptors, and endothelium. Circ Res 94: 1436–1442, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Welch WJ, Ahlstrom NG, Wilcox CS. Mechanism of hypertension during prolonged infusion of thromboxane mimetic. Eur J Intern Med 2: 277–280, 1992 [Google Scholar]
- 51.Welch WJ, Wilcox CS, Folger WH. Mechanism of renal vasoconstriction with thromboxane mimetic: engagement of tubuloglomerular feedback. Adv Prostaglandin Thromboxane Leukot Res 21B: 693–696, 1991 [PubMed] [Google Scholar]
- 52.Wilson SJ, Cavanagh CC, Lesher AM, Frey AJ, Russell SE, Smyth EM. Activation-dependent stabilization of the human thromboxane receptor: role of reactive oxygen species. J Lipid Res 50: 1047–1056, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamagishi T, Yanagisawa T, Taira N. Activation of phospholipase C by the agonist U46619 is inhibited by cromakalim-induced hyperpolarization in porcine coronary artery. Biochem Biophys Res Commun 187: 1517–1522, 1992 [DOI] [PubMed] [Google Scholar]
- 54.Yu JZ, Zhang DX, Zou AP, Campbell WB, Li PL. Nitric oxide inhibits Ca2+ mobilization through cADP-ribose signaling in coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol 279: H873–H881, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Zhang AY, Li PL. Vascular physiology of a Ca2+ mobilizing second messenger–cyclic ADP-ribose. J Cell Mol Med 10: 407–422, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang AY, Yi F, Teggatz EG, Zou AP, Li PL. Enhanced production and action of cyclic ADP-ribose during oxidative stress in small bovine coronary arterial smooth muscle. Microvasc Res 67: 159–167, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Zhang WM, Yip KP, Lin MJ, Shimoda LA, Li WH, Sham JS. ET-1 activates Ca2+ sparks in PASMC: local Ca2+ signaling between inositol trisphosphate and ryanodine receptors. Am J Physiol Lung Cell Mol Physiol 285: L680–L690, 2003 [DOI] [PubMed] [Google Scholar]