The patient was born in a town near Bogotá, Colombia and was adopted in the United States at 2 years of age. At the time of adoption, there was a suspicion of renal disease with failure to thrive. Within a year of arriving in Alabama, she was diagnosed with hypoplastic-dysplastic syndrome. Her renal disease resulted in dialysis at age 16, and she received a living unrelated renal transplant from her adoptive mother at age 18. She was treated with an immunosuppressive regimen that included tacrolimus, mycophenolate, and prednisone. Her immediate posttransplant course was notable only for complaints of mild diarrhea that worsened when her regimen changed from tacrolimus to sirolimus in the 8th month after transplant for a research protocol.
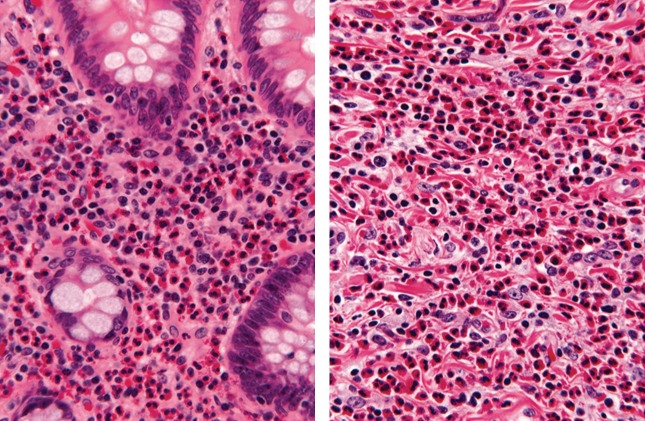
At 9 months posttransplant, she developed severe, constant, right lower quadrant abdominal pain. Her diarrhea worsened and she developed headaches, but she denied having fever, cough, difficulty breathing, vomiting, or bloody stools. A computerized tomography scan showed findings associated with appendicitis, which prompted appendectomy. Pathologic examination of the specimen identified intense transmural eosinophilic infiltration of the appendiceal wall consistent with eosinophilic appendicitis (Figure 1). Biopsies of the terminal ileum, cecum, and colon at the hepatic flexure also revealed eosinophilic infiltrates. No parasitic forms were found in any of the tissue samples by direct visualization. A peripheral eosinophilia of up to 9.5%was documented, and a stool test was negative for ova and parasites (O&P).
Figure 1.
Pathology of appendix. Numerous eosinophils fill the lamina propria (left panel) and infiltrate the submucosa (right panel) of the appendix (hematoxylin and eosin stain, ×132).
After appendectomy, the patient's abdominal pain resolved and her diarrhea improved. However, her eosinophilia persisted, rising as high as 18.3% 3 months later, even after empiric treatment with 2 doses of mebendazole for “intestinal parasite” 1 month after appendectomy. Subsequent evaluation by the gastrointestinal and allergy/immunology services included negative immunoassays for food and upper respiratory allergens, negative screens for celiac disease, negative serology for Toxocara, and a Strongyloides immunoglobulin G (IgG) antibody by enzyme-linked immunosorbent assay (ELISA) that was positive (4.28, reference range <1.00, sensitivity and specificity of 90%; Focus Diagnostics, Cypress, CA). Based on these results, the eosinophilia and gastrointestinal findings were attributed to infection by Strongyloides. The patient was treated with a 4-day course of ivermectin (200 mcg/kg per day) 2 weeks before initial Infectious Diseases (ID) consultation.
In ID clinic, additional history included a positive tuberculin skin test during pretransplant evaluation with a history of Bacillus Calmette-Guérin vaccination, which prompted a 9-month course of isoniazid. The patient reported intermittent “hive-like” rashes in “small red circles” mainly affecting her head, lower extremities, arms, and occasionally buttocks since early childhood, which she attributed to skin allergies. She lived with her parents and with 2 healthy dogs and cats. She reported walking outside barefoot frequently, but she denied any travel outside of the country since arriving in the United States.
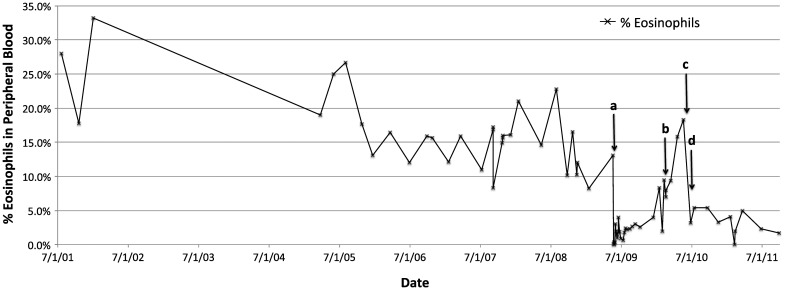
Investigation of the patient's available records revealed negative human immunodeficiency virus status and negative serologies for Trympanosoma cruzi, but testing was not performed for Strongyloides or other parasites on pretransplant evaluation. Any medical evaluation performed in the international adoption process was not available in our patient's medical record. However, her mother denied any medical issues at the time of adoption other than failure to thrive, a suspicion of renal disease, and bow-leggedness. One finding of particular interest was evidence of long-standing (up to 8 years before transplant) significant eosinophilia (>10%) that subsided with transplant but reappeared with the onset of her abdominal symptoms (Figure 2).
Figure 2.
Eosinophil percentage from peripheral blood samples over a 10-year span. Time of transplant (a: 5/21/09), appendectomy (b: 2/18/10), and completion of first (c: 6/5/10) and second (d: 6/27/10) treatments with ivermectin are marked with arrows.
On physical examination the patient was well-appearing and of short stature (143.5 cm, <3% for age), with a soft, nontender abdomen, palpable transplant kidney mass in the lower right quadrant, a well-healed surgical scar over her right abdomen, and no hepatomegaly. No rashes were observed. Although a single stool O&P before initial treatment had been negative, 3 daily stool O&P examinations were repeated and were negative. Serologies for human T-lymphotropic virus (HTLV) and filaria were likewise negative. A complete blood count after the first course of ivermectin revealed that her eosinophilia had improved (white blood cells of 6.27, 3.2% eosinophils).
To ensure eradication in an immunocompromised patient, a second course of ivermectin was given (200 mcg/kg per day for 2 days). At a follow-up visit 6 months later the patient continued to do well with no new symptoms and no further reports of rashes. A repeat Strongyloides IgG level was undetectable.
DISCUSSION
The differential diagnoses for eosinophilic colon disease include inflammatory bowel disease and celiac disease, food or drug allergies (including tacrolimus), rheumatologic conditions, parasitic infections, and primary eosinophil-associated disorders [1]. However, this patient's history, presentation, and negative laboratory and pathologic work-up for noninfectious causes strongly suggested a parasitic infection.
Potential infectious etiologies that can cause appendicitis include Enterobius vermicularis, Ascaris, and other roundworm species such as Strongyloides stercoralis and Trichuris trichiura, Giardia, schistosomes, and Entamoeba histolytica [2]. Appendicitis due to these infections is rare even in tropical settings [3], and those causing eosinophilic intestinal infiltration, such as by S stercoralis and schistosomiasis, are even less common. However, this patient's positive ELISA for Strongyloides and the pathologic findings suggest chronic intestinal strongyloidiasis with associated appendicitis.
Strongyloides stercoralis is a nematode endemic to tropical South America (including Colombia), China, and Southeast Asia, and can also be found in some areas of the southeastern United States. Historically, the prevalence of Strongyloides in the southeastern United States has been reported as high as 3.8% [4]. However, the current degree of endemicity is unknown due to the scarcity of recent comprehensive assessments, the last of which identified a prevalence of 3.0% in southeastern Kentucky schoolchildren in 1982 [5].
The life cycle of S stercoralis in the human host involves 2 different larval stages [6]. The infectious filariform larvae infect humans by penetrating the skin and subsequently migrate to the lungs by bloodstream or lymphatics. In some instances, this can cause the pulmonary symptoms of cough and wheezing seen in Löeffler's syndrome. From there, the larvae ascend up the airway and are swallowed, after which females implant in intestinal walls and lay eggs that cause rhabditiform larvae, which are then released into the environment by stool. Alternatively, a minority of rhabditiform larvae can develop into filariform larvae within the human host intestine that then reinfect the host by penetrating the mucosal wall or the perianal skin. This process, called autoinfection, promotes ongoing cycles of infection within the host and can also cause larva currens, which is a serpiginous pruritic rash typically in the pelvic region that reflects the subcutaneous migration of larvae.
Chronic intestinal Strongyloides infection in normal human hosts can be asymptomatic but can also cause a variety of gastrointestinal symptoms such as pain or diarrhea. Associated findings can include eosinophilia as well as urticarial rashes that are distinct from larva currens. However, in immunocompromised hosts, such as patients receiving steroids or those infected with HTLV-1, parasites can undergo massive proliferation resulting in a hyperinfection syndrome that can include severe pulmonary and gastrointestinal symptoms such as acute respiratory distress syndrome and gastrointestinal obstruction or bleeding. In some cases, infection spreads throughout the body, causing disseminated disease and secondary infections by gram-negative organisms that can lead to sepsis or meningitis. Mortality from hyperinfection has been reported as high as 50% to over 60% in those with disseminated disease [6, 7]. Although such serious disease in renal transplant recipients is uncommon, reported cases typically develop hyperinfection syndrome within 3 months posttransplantation, and these are thought to be due to progression from chronic infection with the initiation of immunosuppression in patients from endemic areas [6]. Ivermectin is the treatment of choice in the immunocompromised host. Because treatment is <100% curative, many experts recommend 2 treatment doses separated by 2 weeks, which is the duration of 1 autoinfection life cycle.
Diagnosing chronic intestinal infection is difficult, as worm burden is often low with unreliable output in stool. Stool examination for larvae has poor sensitivity, although serial exams can improve performance [6]. A single stool examination has been reported to have a sensitivity of 15%–25%, a rate that increases to 50% with 3 serial daily examinations [8]. Alternative methods of stool sample preparation (formalin-ether concentration methods, Harada-Mori filter paper culture, or agar plate culture techniques) and polymerase chain reaction for Strongyloides in stool can improve detection, but they may not be readily available in most laboratories, as is the case at our center.
Isolation of larvae in pathologic specimens, including from the appendix [2, 9], is virtually diagnostic, but negative findings do not rule out infection. Serum ELISA tests for IgG to filariform larvae have good sensitivity and specificity, but they can cross-react with other parasites including Ascaris lumbricoides, filariae, and schistosomes. As a result, the test is particularly useful for those who are not from areas endemic to those infections, but it is less helpful for those who are. In addition, the ELISA test does not distinguish between past and current infection. Titers can be followed to document successful treatment, although it may take up to several months to observe a detectable decrease [6]. A decrease in eosinophilia can also be a useful marker of response to treatment.
Screening for Strongyloides is especially important for patients who are about to undergo significant immunosuppressive therapy, such as prospective renal transplant recipients. Current guidelines recommend pretransplant screening with serologic tests and serial agar-plate stool cultures for patients from endemic areas or with unexplained eosinophilia [10]. Diagnosed cases should receive treatment with ivermectin (200 mcg/kg for 2 days), and empiric treatment should be given to those from areas of high-endemic burden. Some experts recommend a repeat course 2 weeks afterwards. Although rare cases of donor-recipient transmission through the renal graft has been reported [11], at this time only intestine and pancreas donors are recommended for pretransplant screening for Strongyloides [10].
At our center, screening for parasites such as Strongyloides during pretransplant evaluation is targeted based on patient risk factors. Infectious Diseases is not routinely involved in pretransplant evaluation, but is available for consult (as was the case with regards to this patient's positive tuberculin skin test) both before and after transplantation. This case highlights the importance of identifying and screening patients with appropriate risk factors, and further demonstrates the potential benefits of a multidisciplinary approach in the care of transplant patients, including the screening, diagnosis, and treatment of infectious diseases.
Fortunately, this patient never developed signs of hyperinfection syndrome. Concern for this risk in an immunosuppressed patient prompted us to recommend the 2-treatment dose strategy. Although no larval forms were isolated from any of the patient's pathologic specimens, the strongly positive Strongyloides ELISA test in the absence of alternative diagnoses, as well as the subsequent decrease in titers and eosinophilia after treatment with ivermectin, but not mebendazole, is strongly suggestive of Strongyloides infection. It is difficult to assess when such an infection may have been contracted, but the patient's history of early childhood in Colombia as well as her long-standing eosinophilia and ill-defined skin rashes suggest a mild, chronic course that was exacerbated by the initiation of immunosuppressive agents after transplant.
Although gastrointestinal symptoms are common in chronic strongyloidiasis, only a few cases of appendicitis have been attributed to infection by Strongyloides, with only 2 cases reported in children who were siblings not from endemic areas [2, 9, 12]. To our knowledge, this is the first report of appendicitis attributable to Strongyloides infection in a transplant patient, and only the third pediatric case of Strongyloides appendicitis.
In summary, we describe a case of eosinophilic appendicitis with strong evidence of associated Strongyloides infection in a pediatric renal transplant patient adopted from an endemic area. This case demonstrates a rare manifestation of Strongyloides infection and also underscores the importance of screening individuals from endemic areas for parasitic infections before initiating immunosuppressive regimens.
Acknowledgments
We thank the patient and her family who consented to reporting the clinical case, as well as Dr Martin Montes for his advice on this case.
Financial support. This work was supported by the National Institutes of Health (Grant 5K08AI059428-02; to M. S.); the Children's Center for Research and Innovation of the Alabama Children's Hospital Foundation (Grant T0809180003; to M. S.); the Kaul Pediatric Research Initiative of The Children's Hospital of Alabama (Grant T1203060006; to M. S.); and the National Institutes of Health UAB Infectious Diseases Training Grant (5 T32 AI052069; to B. R.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Okpara N, Aswad B, Baffy G. Eosinophilic colitis. World J Gastroenterol. 2009;15:2975–79. doi: 10.3748/wjg.15.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamps LW. Infectious causes of appendicitis. Infect Dis Clin North Am. 2010;24:995–1018. doi: 10.1016/j.idc.2010.07.012. ix–x. [DOI] [PubMed] [Google Scholar]
- 3.Gupta SC, Gupta AK, Keswani NK, et al. Pathology of tropical appendicitis. J Clin Pathol. 1989;42:1169–1172. doi: 10.1136/jcp.42.11.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starr MC, Montgomery SP. Soil-transmitted Helminthiasis in the United States: a systematic review– 1940–2010. Am J Trop Med Hyg. 2011;85:680–84. doi: 10.4269/ajtmh.2011.11-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walzer PD, Milder JE, Banwell JG, et al. Epidemiologic features of Strongyloides stercoralis infection in an endemic area of the United States. Am J Trop Med Hyg. 1982;31:313–19. doi: 10.4269/ajtmh.1982.31.313. [DOI] [PubMed] [Google Scholar]
- 6.Roxby AC, Gottlieb GS, Limaye AP. Strongyloidiasis in transplant patients. Clin Infect Dis. 2009;49:1411–23. doi: 10.1086/630201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeVault GA, Jr, King JW, Rohr MS, et al. Opportunistic infections with Strongyloides stercoralis in renal transplantation. Rev Infect Dis. 1990;12:653–71. doi: 10.1093/clinids/12.4.653. [DOI] [PubMed] [Google Scholar]
- 8.Sato Y, Kobayashi J, Toma H, Shiroma Y. Efficacy of stool examination for detection of Strongyloides infection. Am J Trop Med Hyg. 1995;53:248–50. doi: 10.4269/ajtmh.1995.53.248. [DOI] [PubMed] [Google Scholar]
- 9.Komenaka IK, Wu GC, Lazar EL, Cohen JA. Strongyloides appendicitis: unusual etiology in two siblings with chronic abdominal pain. J Pediatr Surg. 2003;38:E8–10. doi: 10.1016/s0022-3468(03)00415-9. [DOI] [PubMed] [Google Scholar]
- 10.Kotton CN, Lattes R. Parasitic infections in solid organ transplant recipients. Am J Transplant. 2009;9(Suppl 4):S234–51. doi: 10.1111/j.1600-6143.2009.02915.x. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton KW, Abt PL, Rosenbach MA, et al. Donor-derived Strongyloides stercoralis infections in renal transplant recipients. Transplantation. 2011;91:1019–24. doi: 10.1097/TP.0b013e3182115b7b. [DOI] [PubMed] [Google Scholar]
- 12.Cruz DB, Friedrisch BK, Fontanive Junior V, da Rocha VW. Eosinophilic acute appendicitis caused by Strongyloides stercoralis and Enterobius vermicularis in an HIV-positive patient. BMJ Case Rep. 2012:2012. doi: 10.1136/bcr.01.2012.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]