Abstract
Human embryonic stem cells (hESCs) are uniquely endowed with a capacity for both self-renewal and multilineage differentiation. The aim of this investigation was to determine if short periods of cyclic mechanical strain enhanced dexamethasone, ascorbic acid, and β-glycerophosphate (triple-supplement)-induced osteogenesis and bone nodule formation by hESCs. Colonies were cultured for 21 days and divided into control (no stretch) and three treatment groups; these were subjected to in-plane deformation of 2% for 5 s (0.2 Hertz) every 60 s for 1 h on alternate days in BioFlex plates linked to a Flexercell strain unit over the following periods (day 7–13), (day 15–21), and (day 7–21). Numerous bone nodules were formed, which stained positively for osteocalcin and type I collagen; in addition, MTS assays for cell number as well as total collagen assays showed a significant increase in the day 7–13 group compared to controls and other treatment groups. Alizarin Red staining further showed that cyclic mechanical stretching significantly increased the nodule size and mineral density between days 7–13 compared to control cultures and the other two experimental groups. We then performed a real-time polymerase chain reaction (PCR) microarray on the day 7–13 treatment group to identify mechanoresponsive osteogenic genes. Upregulated genes included the transcription factors RUNX2 and SOX9, bone morphogenetic proteins BMP1, BMP4, BMP5, and BMP6, transforming growth factor-β family members TGFB1, TGFB2, and TGFB3, and three genes involved in mineralization—ALPL, BGLAP, and VDR. In conclusion, this investigation has demonstrated that four 1-h episodes of cyclic mechanical strain acted synergistically with triple supplement to enhance osteogenesis and bone nodule formation by cultured hESCs. This suggests the development of methods to engineer three-dimensional constructs of mineralized bone in vitro, could offer an alternative approach to osseous regeneration by producing a biomaterial capable of providing stable surfaces for osteoblasts to synthesize new bone, while at the same time able to be resorbed by an osteoclastic activity—in other words, one that can recapitulate the remodeling dynamics of a naturally occurring bone matrix.
Introduction
Human embryonic stem cells (hESCs) isolated from the inner cell mass of preimplantation blastocyst-stage embryos are uniquely endowed with a capacity for both self-renewal and multilineage differentiation.1 Self-renewal makes hESCs particularly useful in regenerative medicine because in theory, it could provide an unlimited supply of donor cells for transplantation,2 and hESCs can be frozen and thawed with a relatively high survival rate.3 Osteogenic lineages derived from hESCs therefore have considerable potential for clinical applications in bone tissue engineering and reconstructive surgery, as well as providing useful experimental models for studying osteogenesis and skeletal development.4
During the 1970–80s, much effort went into understanding the mechanisms regulating the differentiation of mesenchymal stem cells (MSCs) into specialized lineages using cultured bone marrow stromal cells5,6 and cells isolated from fetal rat calvaria.7,8 These investigations established the ability of dexamethasone, ascorbic acid, and β-glycerophosphate (triple supplement) to promote osteoblast differentiation and mineralized bone nodule formation in vitro, thereby providing the methodology for the first reports on the directed commitment of hESCs to the osteoblast lineage.4,9
While mechanical loading is recognized as an important determinant of bone mass and architecture, the mechanically induced strain can have negative as well as positive effects on bone. Increases in bone strains above a certain critical threshold have a positive effect on bone mass, while reductions in strain magnitude lead to bone loss and osteopenia.10 This means that cultured bone cells deprived of mechanical stimuli are effectively in a physiological default state. The sensitivity of bone cells to their biomechanical environment has been widely investigated with a variety of in vitro models,11 and short-time mechanical strain and dexamethasone have both been shown to promote osteogenic differentiation by human bone marrow stromal cells (hBMSCs12,13). However, a role for mechanical strain in the induction or enhancement of osteoblast differentiation from hESCs is yet to be investigated.
Human ESC differentiation is a precisely controlled process in which, specific germ layer marker expression is regulated spatially and temporally.14 To differentiate into osteoblasts, hESCs must first pass through an intermediate osteoprogenitor cell stage for which, various methods have been reported.15–18 The aim of the present investigation therefore was to determine if short periods of cyclic mechanical strain were able to enhance triple-supplement directed osteogenesis and bone nodule formation by hESCs and the optimal time when it might be most effective.
Materials and Methods
Culture of hESCs
H1 hESCs (WiCell Research Institute, Madison, WI) were used in all experiments; mouse embryonic fibroblasts (MEFs) were used as feeder layers for hESC culture. Briefly, MEF cells were extracted from 12.5-day uteri of pregnant CF1 mice and cultured to passage 4 before inactivation with 0.01 mg/mL mitomycin C (Kyowa Hakko Kogyo) treatment for 2 h. The MEF medium was composed of the DMEM high glucose (Sigma-Aldrich) and 10% FBS (fetal bovine serum; Biowest). About 1.2×106 inactivated MEF cells were seeded onto 0.1% gelatin-coated six-well plates 24 h before hESC passage; The hESC medium was composed of the DMEM-F12 (Gibco, Life Technologies), 20% knockout serum replacement (Gibco) supplemented with 4 ng/mL FGF-2 (Invitrogen), 1 mM L-glutamine (Gibco), 1% nonessential amino acid (NEAA; Gibco), and 0.1 mM β-mercaptoethanol (2-ME; Sigma-Aldrich). Subconfluent hESC cultures were passaged every 5–6 days following 1 mg/mL collagenase type IV treatment for 5 min and mechanical scrapping. All cells were cultured at 37°C in a humidified atmosphere of 5% CO2/95% air.
Immunocytochemical staining for pluripotency of hESCs; colocalization of osteocalcin and type I collagen in osteogenic nodules
Cell cultures were fixed with 0.5 mL 4% paraformaldehyde (Sigma) for 15 min at room temperature (RT), permeabilization for 10 min with 0.2% Triton X-100 in phosphate-buffered saline (PBS), and blocked for 1 h with 5% goat serum and 2% BSA (Sigma-Aldrich) in PBS. The primary antibody was then incubated with the cells at 4°C overnight. After three washes with PBS, the respective secondary antibody incubated with the cells for 2 h at RT. DAPI was added 10 min before the last three washes with PBS and examined by fluorescent microscopy (Olympus IX70).
The primary antibodies used were mouse anti-human SSEA4 (Santa Cruz, Biotechnology); mouse anti-human Oct4 (Santa Cruz Biotechnology); rabbit anti-human Col-I (AbD Serotec); mouse anti-human osteocalcin (AbD Serotec). Mouse IgG1 Isotype Control (R&D systems) and Rabbit polyclonal IgG (Abcam) were used as isotype control, respectively, for staining. The secondary antibodies used were Alexa fluor 488 goat anti-rabbit IgG (Invitrogen) and Alexa Fluor 594 goat anti-mouse IgG (Invitrogen).
Osteogenic differentiation of hESCs under cyclic mechanical strain
H1 hESC colonies were treated with 2 mg/mL collagenase IV for 30 min to detach them from the feeder layers. Floating colonies were collected and split into smaller colonies through vigorous pipetting in the triple-supplement osteogenic medium composed of the DMEM high glucose (Sigma-Aldrich), 10% FBS (Fetal bovine serum; Biowest) supplemented with 10−7M dexamethasone, 2×10−4 M ascorbic acid, and 10 mM β-glycerophosphate (Sigma-Aldrich).
Equal numbers of colonies (approximately 0.5×106 cells) were then seeded into six-well BioFlex plates coated with type I collagen (Flexcell International) and cultured in the osteogenic medium at 37°C in a humidified atmosphere of 5% CO2/95% air. Colonies were cultured for 21 days and divided into four groups: the control group (no stretch to the cultured cells) and three treatment groups stretched over the following periods (day 7–13), (day 14–21), and (day 7–21). Treatment groups were subjected to an episode of in-plane equibiaxial deformation of 2% for 5 s (0.2 Hertz) every 60 s for 1 h every other day in BioFlex plates linked to a FX-4000 Flexercell Tension Plus strain unit (Flexcell International). Three biological replicates were cultured and assayed for each analytical method.
MTS assay
Cell proliferation was measured by the MTS assay19 based on the ability of MTS in the presence of phenazine methosulfate (PMS) to give a water-soluble formazan product. Briefly, 120 μL of the MTS reagent (Promega) was mixed with 600 μL of media and incubated for 4 h in darkness with gentle agitation in cultured cells. Reagents were also added into blank wells for background control. Hundred microliters of the purple colored media was collected and optical density read at 490 nm with an Infinite 200 microplate reader (Tecan). A standard curve based on the optical density of human fetal osteoblasts (CRL11372) was plotted and the number of cells calculated from the standard curve.
Measurement of total collagen deposition
Total collagen synthesis was quantified by a Sircol soluble collagen assay kit (Biocolor) with some modification. Briefly, cell cultures were washed three times with PBS and 500 μL of the Sircol dye reagent added to each well. Plates were gently agitated for 1 h followed by another three washes with chilled acid-salt wash reagent to remove unbound dye. Five hundred microliters of alkaline reagents was then added to each well to extract bound dye for 10 min with gentle agitation. Absorbance of 200 μL of the extracted dye solution was taken at 555 nm with an Infinite 200 microplate reader (Tecan).
Alizarin Red staining
Alizarin Red staining was used to identify calcium by the formation of Alizarin Red S-calcium complexes. At the end of 21 days, the cells were washed three times with Ca2+- and Mg2+-free PBS and fixed with 4% paraformaldehyde for 15 min at RT. The plates were then washed with double-distilled water (dd-H2O) three times before staining with 500 μL of 2% Alizarin Red solution for 5 min at RT in the dark. Plates were then rinsed thoroughly with dd-H2O to remove nonspecific staining, placed under ventilation for 1 day to dry out, and photographed using an inverted microscope (Olympus IX70). Eight pictures, taken at 40× magnification after Alizarin Red staining, were randomly selected, nodules and areas positively stained were analyzed from each group using the software ImageJ. (NIH).
RT-PCR microarray for osteogenic marker expression
Total mRNAs were extracted from the 21-day cultures using an RNeasy Kit (Qiagen). The concentration and purity (A260/A280) of the samples was determined with a NanoDrop ND-1000 spectrophotometer (NanoDrop). RNA integrity was checked by running 1 μg of a mRNA sample through 1% agarose in a 1× TBE buffer (Tris/Borate/EDTA; First Base). cDNAs were synthesized using the RT2 First Strand Kit (SABiosciences). Briefly, 1 μg of each RNA sample was incubated with genomic DNA elimination mixture for 5 min at 42°C and immediately chilled on ice for 1 min. The mRNA samples were then mixed with RT first-strand kit master mix and enzyme and incubated at 42°C for 15 min and stopped immediately by heating at 95°C for 5 min. One microliter of cDNA was used for each well of Human Osteogenesis RT2 Profiler PCR array plates (PAHS-026; SABiosciences, Qiagen). The plates were loaded into a StepOne Real-Time PCR Instrument (Applied Biosystems) for 40 cycles (15 s at 95°C and 1 min at 60°C each cycle). Results were analyzed by web-based polymerase chain reaction (PCR) array data analysis software provided by the manufacturer.
Statistical analysis
Statistical analyses were performed using an SPSS software package (IBM). Differences between groups were evaluated by the Student's t-test (two-tailed) and one-way ANOVA with the level of significance set at p<0.05.
Results
The pluripotency of the hESC colonies was established by two criteria: first, by the morphology of the colonies, which should have distinct boundaries demarcating them from the MEF feeder layer (Fig. 1A). Second, by immunocytochemical staining for SSEA4, a surface marker for embryonic stem cells (Fig. 1B) and Oct4, a transcription factor regulating hESC pluripotency (Fig. 1C). The colonies reacted positively for both pluripotent cell surface marker expression and transcriptional activation.
FIG. 1.
(A) Phase-contrast image of an expanding human embryonic stem cell (hESC) colony shows a clear boundary from surrounding mouse embryonic fibroblasts feeder cells. (B, C) Immunocytochemical staining of pluripotent hESC transcriptional regulator Oct4 (Green) and surface marker SSEA4 (Red) confirms the pluripotency of cells before induction of differentiation. Upon differentiation, immunocytochemical staining for osteocalcin (Green) and type I collagen (Red) was performed to examine the colocalization of these two bone matrix components in the control group (D, E) and treatment group day 7–13 (F, G). Cells were counter stained with DAPI (Blue) for the nucleus. Cell density was clearly observed at the peripheral part nodules and osteocalcin and collagen components were observed to be located in the center of the nodule. Scale bars represent 500 μm. Color images available online at www.liebertpub.com/tea
Immunocytochemical staining of the nodules in the control group and treatment groups for osteocalcin (Fig. 1D, F) and type I collagen (Fig. 1E, G) revealed that osteocalcin and type I collagen were colocalized to the center, whereas the majority of the cells were located at the periphery. Analyses of Alizarin Red staining (Fig. 2A–D) further showed that cyclic mechanical stretching significantly increased the mineral density (Fig. 2E) and nodule size (Fig. 2F) between days 7–13 compared to control cultures and the other two experimental groups.
FIG. 2.
Photomicrographs of cultures stained with Alizarin Red after 21 days. (A, B) The control group showed positive Ca2+ stain and bone nodule formation. (C, D) The D7–13 group showed the best induction of osteogenesis and nodule formation following short-term cyclic strain. Red indicates positive staining for Ca2+ in the matrix; black indicates crystalline hydroxyapatite. Histomorphological analyses for eight randomly selected photos from each group were analyzed for the positively stained area using ImageJ. (E) The treatment groups showed a significantly higher mineralized area than the control group. Among all treatment groups, day 7–13 showed more than 80% positive mineralized area in a randomly selected field. Again, sizes of nodules from eight randomly selected photos from each group were analyzed using ImageJ. (F) Nodules from the treatment groups are generally larger than control groups. Among all treatment groups, day 7–13 showed more than 60% positive mineralized nodule areas in the randomly selected field. Size bars A, C represent 2 mm and B, D 500 μm. (**p<0.01, *p<0.05). Color images available online at www.liebertpub.com/tea
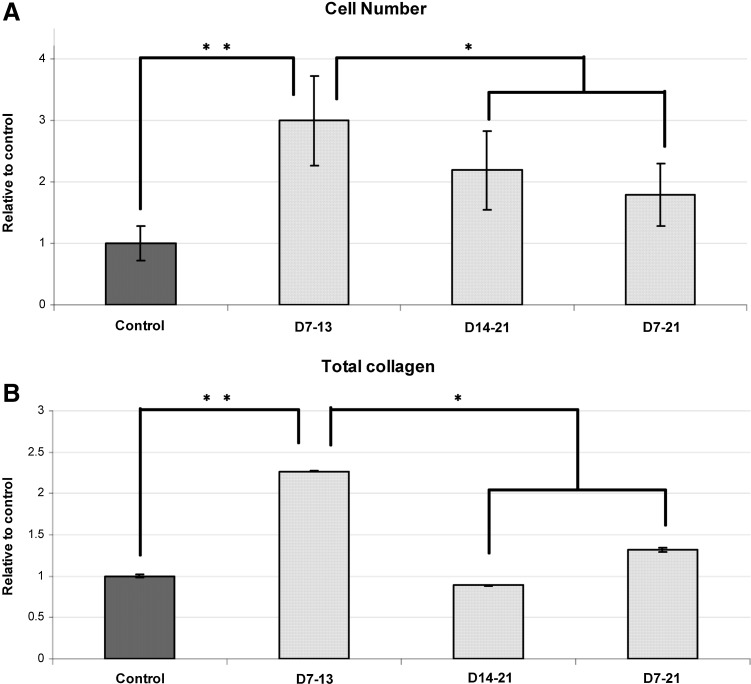
At day 21 confluent culture, the MTS assay showed that intermittent substrate deformation resulted in a significant increase in cell proliferation and total cell number in all treatment groups compared to controls. The most significant increase was found in group day 7–13, where the cell number increased up to threefold from the control (Fig. 3A). Total collagen build up was also significantly higher than in treatment groups day 14–21 and day 7–21, as well as the control group (Fig. 3B).
FIG. 3.
(A) All groups were cultured for 21 days when the total cell number was measured by the MTS assay. All three treatment groups were significantly higher than controls; cell numbers in treatment groups day 15–21 and day 7–21 were significantly lower than day 7–13. (B) Total collagen at day 21. The treatment group day 7–13 shows a significantly higher total collagen deposition in the extracellular matrix compared with all other groups. There was no significant difference between the control group and the other two treatment groups. (n=3). *p<0.05 **p<0.01.
In view of the finding that the day 7–13 culture group was the most responsive to mechanically induced strain, we then performed a real-time PCR osteogenesis microarray to identify any mechanoresponsive osteogenic genes in this group compared to controls (Table 1). Selected upregulated genes included the transcription factors RUNX2 and SOX9, five bone morphogenetic proteins BMP1, BMP4, BMP5, and BMP6, three transforming growth factor-β family members TGFB1, TGFB2, and TGFB3, and three genes that play key roles in mineralization—ALPL, BGLAP (osteocalcin), and VDR.
Table 1.
Mechanoresponsive Genes Significantly Upregulated in Treatment Group Day 7–13 Relative to Controls
| HGNC agreed gene symbol | Description | Fold upregulation |
|---|---|---|
| ALPL | Alkaline phosphatase, liver/bone/kidney | 180.30 |
| BGLAP | Bone γ-carboxyglutamate (gla) protein (Osteocalcin) | 168.60 |
| BMP1 | Bone morphogenetic protein 1 | 20.42 |
| BMP4 | Bone morphogenetic protein 4 | 21.74 |
| BMP5 | Bone morphogenetic protein 5 | 34.29 |
| BMP6 | Bone morphogenetic protein 6 | 26.31 |
| GDF10 | Growth differentiation factor 10 | 896.43 |
| RUNX2 | Runt-related transcription factor 2 | 469.43 |
| SMAD2 | SMAD family member 2 | 138.71 |
| SOX9 | SRY (sex determining region Y)-box 9 | 92.76 |
| TGFB1 | Transforming growth factor, beta 1 | 11.35 |
| TGFB2 | Transforming growth factor, beta 2 | 11.41 |
| TGFB3 | Transforming growth factor, beta 3 | 56.90 |
| VDR | Vitamin D (1,25-dihydroxyvitamin D3) receptor | 88.10 |
HGNC, Human Genome Organization Gene Nomenclature Committee.
Discussion
An important early step in skeletal development is the condensation and differentiation of MSCs into cells of the osteoblast lineage—these synthesize a complex extracellular matrix composed of collagens, noncollagenous proteins, and glycoproteins, which rapidly becomes mineralized to form an ossification center.20 Previous research from this laboratory has shown that hESCs cultured in a cocktail of dexamethasone, ascorbic acid, and β-glycerophosphate formed morphologically distinct nodule-like structures containing osteogenic cells.4 That the high-density condensed cellular mass within the nodules was conducive to osteogenesis and the formation of a mineralized matrix, was confirmed in the present study by the immunolocalization of osteocalcin and type I collagen, and the development of Alizarin Red S–calcium complexes.
Bone cells are acutely sensitive to their biomechanical environment and the importance of mechanical signaling in the maintenance of the osteoblast phenotype and bone mass is well established.11 Evidence from this study based on previous reports in which bone marrow stromal cells were used as a source of hMSCs,12,13 shows that only short bursts of cyclic mechanical deformation were required to enhance triple-supplement directed osteoblast differentiation and osteogenesis by hESCs. The advantage of hESCs in regenerative medicine is they represent a pure stem cell population, unlike MSCs, which are characterized by cellular heterogeneity, lineage restriction, and relatively few multipotent stem cells.21,22 Heterogeneity within individual MSC preparations is an additional disadvantage, and the likely explanation for the high variance in BGLAP and RUNX2 gene expression reported in mechanically strained hBMSC cultures.12
An equibiaxial cyclic mechanical strain of 2% not only stimulated bone cell proliferation, but also extracellular matrix formation, and was found to be most effective when applied as four 1-h episodes from 7–13 days of the 21-day culture period. This demonstrates that relatively low substrate deformation acting over a very short time scale is able to exert a significant effect, and is in keeping with the findings of in vivo experiments showing that a substantial osteogenic response can be achieved in functionally isolated bones after remarkably few cycles of dynamic loading.23 One question that is frequently asked, but is not possible to answer adequately is the strain profile of the cells. In contemporary cell culture systems when a substrate is deformed, irrespective of whether in-plane or out-of-plane, uniaxial or biaxial, the cells will reorientate and be exposed to a combination of tensile, compressive, and shear strains; the amount of deformation will also vary with the position of the cells within the field, and in the Flexercell system, it has been estimated the cells will only experience about half the deformation programmed into the computer.24,25 An added complication of the present cultures is that cells are encapsulated both within the three-dimensional (3D) nodules, and attached as a monolayer to the two-dimensional type I collagen-coated substrate. In any event, the question of the precise strain may well be academic, because the deformation profile of cells in vivo is likely to be equally complex, and classical experimental studies in animal models have shown that bone is insensitive to static strain and appears unable to distinguish between cyclic tensile and compressive strain in terms of an osteogenic response.26,27
We have a much better understanding of how the mechanical signal is translated into a biological one, a field that has been investigated in osteoblastic cells for more than three decades—these have highlighted the complexity of the mechanisms involved and shown that mechanical stress like cytokines and growth factors can activate multiple cell-signaling pathways.28 The earliest experiments found the immediate response to mechanical strain was generation of prostaglandins (PGs), and the second messengers cAMP and inositol phosphates.29–31 Changes in intracellular [Ca2+] were later shown to occur via stretch-activated ion channels.32,33 However, activation of these pathways lies downstream from the initial mechanoreception event at focal adhesions, where firm attachment is necessary for mechanical distortion to be recognized.34,35 Cells in culture are not uniformly attached to their substrate, but are tack-welded at focal adhesions, sites where integrins physically link actin-associated cytoskeletal proteins (talin, vinculin, α-actinin, paxillin) with the structural macromolecules of the extracellular matrix such as collagen, laminin, and fibronectin,36 as well as to adhesion molecules on the surface of adjacent cells. Integrins are therefore ideally placed to mediate the transmission of bidirectional forces across the cell membrane,37 playing key roles in cell proliferation, differentiation, and migration, as well as adhesion-induced (outside-in) changes in cell physiology.34,38
The RT-PCR microarray carried out on the day 7–13 culture group showed that two genes essential for skeletal development, the osteoblast-specific transcription factor RUNX2 and its chondrogenic counterpart SOX9, were both upregulated. The RUNX2 protein binds to and regulates the expression of multiple genes, including those for osteocalcin, type I collagen, and osteopontin,39 and is necessary for both endochondral and membranous bone formation.40,41 RUNX2 was the highest upregulated gene; this is in striking contrast, however, to a recent study, which reported that continuous mechanical strain downregulated RUNX2 expression and alkaline phosphatase activity in hMSCs,42 but as mentioned earlier, this is likely to be related to the inability of bone to respond positively to a static strain.26
BMPs are members of the TGF-β gene superfamily and are important downstream determinants of the osteoblast phenotype. Originally discovered by their ability to induce ectopic cartilage and bone formation in vivo, purification of the BMP activity from bovine bone led to the identification of seven novel human proteins labeled BMP-1 to BMP-7 with multiple biological activities, not all of them osteogenic.43 BMPs are expressed at sites of skeletogenesis, where they first induce chondrogenesis making them to some extent also cartilage morphogenetic proteins and explaining why SOX9, a transcription factor that directly regulates the type II collagen gene, the major structural protein in the cartilage matrix,44 was expressed in the cultures. TGFB1, TGFB2, and TGFB3, which encode multifunctional peptides controlling proliferation, differentiation, and other functions, were also upregulated, as was SMAD2. SMADs mediate activin, TGF-β, and BMP signaling from receptors to nuclei.
The VDR, BGLAP, and ALPL genes are all involved at some stage in matrix mineralization. The vitamin D receptor is an intracellular receptor that specifically binds 1,25(OH)2vitaminD3 (calcitriol) and mediates its effects, which includes the induction of bone Gla protein (osteocalcin) synthesis, the most abundant noncollagenous protein in bone.45 A distinguishing feature of this osteoblast-specific protein is that it undergoes vitamin K-dependent, post-translational carboxylation of its glutamic acids (at positions 17, 21, and 24 in all species) to form three γ-carboxyglutamic acid (Gla) residues, which have high affinity for mineral ions.46 As a result, most newly synthesized osteocalcin remains in bone bound to hydroxyapatite. Once regarded as a somewhat uninteresting matrix protein of limited function, osteocalcin has recently achieved hormone status and emerged as part of a complex signaling network between bone, the pancreas, and adipose tissue, two organs more usually associated with the regulation of energy metabolism.47 Another important regulator of matrix mineralization is ALP, a membrane-bound enzyme consisting of several isoforms that hydrolyzes various monophosphate esters at high pH. Osteoblasts promote matrix mineralization by budding off matrix vesicles, extracellular organelles containing ALP, ATPase, inorganic pyrophosphate, and proteinases such as the plasminogen activators, which act as seeding sites for hydroxyapatite crystal growth.48 Type I collagen, by binding and orientating proteins such as osteonectin that nucleate hydroxyapatite, provides an additional mechanism.
In conclusion, this investigation has demonstrated that short periods of cyclic mechanical strain act synergistically with dexamethasone, ascorbic acid, and β-glycerophosphate to enhance osteogenesis and bone nodule formation by cultured hESCs. While the use of osteogenic lineages derived from hESCs has considerable potential in bone tissue engineering, current strategies focusing on the building of in vitro bone constructs arising from cells in a physiologically default state have been disappointing. What is required is a material that can recapitulate the remodeling dynamics of naturally occurring bone matrix in 3D culture systems capable of being mechanically loaded. This offers a different and challenging approach to the regeneration of bone, while at the same time avoiding the potential hazards associated with banked bone. We have found that after mechanical loading, both the cell number and mineralization density were significantly higher than in the control group, where cells were not mechanically loaded, suggesting that the cell number is positively related with nodule formation. Mechanical strain, especially applied at days 7–13, was able to maximize the confluency of osteoblast lineage cells and nodule formation from hESCs. Bone constructs generated from this protocol will benefit future stem cell therapy by providing a novel method for generating osteoblasts, and at the same time incorporating them into their autologous bone matrix.
Acknowledgments
This research was partially funded by the following grants from Singapore Ministry of Education: R221-000-023-112, R221-000-024-133 and National University Health System: R221000053515.
Disclosure Statement
No competing financial interests exist.
References
- 1.Thomson J.A. Itskovitz-Eldor J. Shapiro S.S. Waknitz M.A. Swiergiel J.J. Marshall V.S. Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Heng B.C. Cao T. Stanton L.W. Robson P. Olsen B. Strategies for directing the differentiation of stem cells into the osteogenic lineage in vitro. J Bone Miner Res. 2004;19:1379. doi: 10.1359/JBMR.040714. [DOI] [PubMed] [Google Scholar]
- 3.Heng B.C. Clement M.V. Cao T. Caspase inhibitor Z-VAD-FMK enhances the freeze-thaw survival rate of human embryonic stem cells. Biosci Rep. 2007;27:257. doi: 10.1007/s10540-007-9051-2. [DOI] [PubMed] [Google Scholar]
- 4.Cao T. Heng B.C. Ye C.P. Liu H. Toh W.S. Robson P. Li P. Hong Y.H. Stanton L.W. Osteogenic differentiation within intact human embryoid bodies result in a marked increase in osteocalcin secretion after 12 days of in vitro culture, and formation of morphologically distinct nodule-like structures. Tissue Cell. 2005;37:325. doi: 10.1016/j.tice.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Friedenstein A.J. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- 6.Owen M. Marrow stromal stem cells. J Cell Sci. 1988;10:63. doi: 10.1242/jcs.1988.supplement_10.5. [DOI] [PubMed] [Google Scholar]
- 7.Bellows C.G. Aubin J.E. Heersche J.N.M. Antosz M.E. Mineralized bone nodules formed in vitro from enzymatically released rat calvarial cell populations. Calcif Tissue Int. 1986;38:143. doi: 10.1007/BF02556874. [DOI] [PubMed] [Google Scholar]
- 8.Grigoriadis A.E. Heersche J.N.M. Aubin J.E. Differentiation of muscle, fat, cartilage, and bone from progenitor cells present in a bone-derived clonal cell population: effect of dexamethasone. J Cell Biol. 1986;106:2139. doi: 10.1083/jcb.106.6.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sottile V. Thomson A. McWhir J. In vitro osteogenic differentiation of human ES cells. Cloning Stem Cells. 2003;5:149. doi: 10.1089/153623003322234759. [DOI] [PubMed] [Google Scholar]
- 10.Frost H.M. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219:1. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 11.Rubin J. Rubin C. Jacobs C.R. Molecular pathways mediating mechanical signaling in bone. Gene. 2006;367:1. doi: 10.1016/j.gene.2005.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jagodzinski M. Drescher M. Zeichen J. Hankemeier S. Krettek C. Bosch U. van Griensven M. Effects of cyclic longitudinal mechanical strain and dexamethasone on osteogenic differentiation of human bone marrow stromal cells. Eur Cell Mater. 2004;7:35. doi: 10.22203/ecm.v007a04. [DOI] [PubMed] [Google Scholar]
- 13.Sittichokechaiwut A. Edwards J.H. Scutt A.M. Reilly G.C. Short bouts of mechanical loading are as effective as dexamethasone at inducing matrix production by human bone marrow mesenchymal stem cells. Eur Cell Mater. 2010;20:45. doi: 10.22203/ecm.v020a05. [DOI] [PubMed] [Google Scholar]
- 14.Pekkanen-Mattila M. Pelto-Huikko M. Kujala V. Suuronen R. Skottman H. Aalto-Setälä K. Kerkelä E. Spatial and temporal expression pattern of germ layer markers during human embryonic stem cell differentiation in embryoid bodies. Histochem Cell Biol. 2010;133:595. doi: 10.1007/s00418-010-0689-7. [DOI] [PubMed] [Google Scholar]
- 15.Wu R. Gu B. Zhao X. Tan Z. Chen L. Zhu J. Zhang M. Derivation of multipotent nestin +/CD271−/STRO-1−mesenchymal-like precursors from human embryonic stem cells in chemically defined conditions. Hum Cell. 2013;26:19. doi: 10.1007/s13577-011-0022-3. [DOI] [PubMed] [Google Scholar]
- 16.Lian Q. Lye E. Suan. Yeo K. Tan E.K.W. Salto-Tellez M. Liu T.M. Palanisamy N. Oakley R.M.E. Lee E.H. Lim B. Lim S.-K. Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cells. 2007;25:425. doi: 10.1634/stemcells.2006-0420. [DOI] [PubMed] [Google Scholar]
- 17.Barberi T. Willis L.M. Socci N.D. Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:e161. doi: 10.1371/journal.pmed.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee E.J. Lee H.N. Kang H.J. Kim K.H. Hur J. Cho H.J. Lee J. Chung H.M. Cho J. Cho M.Y. Oh S.K. Moon S.Y. Park Y.B. Kim H.S. Novel embryoid body-based method to derive mesenchymal stem cells from human embryonic stem cells. Tissue Eng Part A. 2010;16:705. doi: 10.1089/ten.tea.2008.0596. [DOI] [PubMed] [Google Scholar]
- 19.Cory A.H. Owen T.C. Barltrop J.A. Cory J.G. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Comm. 1991;3:207. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 20.Hall B.K. Miyake T. All for one and one for all: condensation and the initiation of skeletal development. BioEssays. 2000;22:138. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Jukes J.M. van Blitterswijk C.A. de Boer J. Skeletal tissue engineering using embryonic stem cells. J Tissue Eng Regen Med. 2010;4:165. doi: 10.1002/term.234. [DOI] [PubMed] [Google Scholar]
- 22.Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther. 2010;21:1045. doi: 10.1089/hum.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin C.T. Lanyon L.E. Regulation of bone formation by applied dynamic loads. J Bone Joint Surg. 1984;66A:397. [PubMed] [Google Scholar]
- 24.Vande Geeste J.P. Di Martino E.S. Vorp D.A. An analysis of the complete strain field within Flexercell membranes. J Biomech. 2004;40:173. doi: 10.1016/j.jbiomech.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 25.Wall M.E. Weinhold P.S. Siu T. Brown T.D. Banes A.J. Comparison of cellular strain with applied substrate strain in vitro. J Biomech. 2007;40:173. doi: 10.1016/j.jbiomech.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 26.Hěrt J. Liśková M. Landa J. Reaction of bone to mechanical stimuli. Part 1. Continuous and intermittent loading of tibia in rabbit. Folia Morphologica. 1971;19:290. [PubMed] [Google Scholar]
- 27.Lanyon L.E. Rubin C.T. Static v dynamic loads as an influence on bone remodelling. J Biomech. 1984;17:897. doi: 10.1016/0021-9290(84)90003-4. [DOI] [PubMed] [Google Scholar]
- 28.Liedert A. Kaspar D. Blakytny R. Claes L. Ignatius A. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Comm. 2006;349:1. doi: 10.1016/j.bbrc.2006.07.214. [DOI] [PubMed] [Google Scholar]
- 29.Harell A. Dekel S. Binderman I. Biochemical effect of mechanical stress on cultured bone cells. Calcif Tissue Res. 1977;22:202. doi: 10.1007/BF02064065. [DOI] [PubMed] [Google Scholar]
- 30.Yeh C.-K. Rodan G.A. Tensile forces enhance prostaglandin E 2 synthesis in osteoblastic cells grown on collagen ribbons. Calcif Tissue Int. 1984;36:S67. doi: 10.1007/BF02406136. [DOI] [PubMed] [Google Scholar]
- 31.Sandy J.R. Meghji S. Farndale R.W. Meikle M.C. Dual elevation of cyclic AMP and inositol phosphates in response to mechanical deformation of murine osteoblasts. Biochim et Biophys Acta. 1989;1010:265. doi: 10.1016/0167-4889(89)90171-7. [DOI] [PubMed] [Google Scholar]
- 32.Davidson R.M. Tatakis D.W. Auerbach A.L. Multiple forms of mechanosensitive channels in osteoblast-like cells. Pflugers Archiv. 1990;416:646. doi: 10.1007/BF00370609. [DOI] [PubMed] [Google Scholar]
- 33.McDonald F. Somasundaram B. McCann T.J. Mason W.T. Meikle M.C. Calcium waves in fluid flow stimulated osteoblasts are G protein mediated. Arch Biochem Biophys. 1996;326:31. doi: 10.1006/abbi.1996.0043. [DOI] [PubMed] [Google Scholar]
- 34.Wang N. Butler J.P. Ingber D.E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;269:1124. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 35.DeMali K.A. Wennerberg K. Burridge K. Integrin signaling to the actin cytoskeleton. Current Opin Cell Biol. 2003;15:72. doi: 10.1016/s0955-0674(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 36.Sastry S.K. Burridge K. Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp Cell Res. 2000;261:25. doi: 10.1006/excr.2000.5043. [DOI] [PubMed] [Google Scholar]
- 37.Ingber D.E. Integrins as mechanochemical transducers. Current Opin Cell Biol. 1991;3:841. doi: 10.1016/0955-0674(91)90058-7. [DOI] [PubMed] [Google Scholar]
- 38.Clarke E.A. Brugge J.S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 39.Ducy P. Zhang R. Geoffroy V. Ridall A.L. Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 40.Otto F. Thornell A.P. Crompton T. Denzel A. Gilmour K.C. Rosewell I.R. Stamp G.W. Beddington R.S. Mundlos S. Olsen B.R. Selby P.B. Owen M.J. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 41.Komori T. Yagi H. Nomura S. Yamaguchi A. Sasaki K. Deguchi K. Shimizu Y. Bronson R.T. Gao Y.H. Inada M. Sato M. Okamoto R. Kitamura Y. Yoshiki S. Kishimoto T. Targeted disruption of Cbfa-1 results in a complete lack of bone owing to maturational arrest of osteoblasts. Cell. 1997;89:755. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 42.Shi Y. Li H. Zhang X. Fu Y. Huang Y. Lui P.P. Tang T. Dai K. Continuous cyclic mechanical tension inhibited Runx2 expression in mesenchymal stem cells through Rho-ERK1/2 pathway. J Cell Physiol. 2011;226:2159. doi: 10.1002/jcp.22551. [DOI] [PubMed] [Google Scholar]
- 43.Wozney J.M. Rosen V. Celeste A.J. Mitsock L.M. Whitters M.J. Kriz R.W. Hewick R.M. Wang E.A. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 44.Bell D.M. Leung K.K. Wheatley S.C. Ng L.J. Zhou S. Ling K.W. Sham M.H. Koopman P. Tam P.P. Cheah K.S. SOX9 directly regulates the type-II collagen gene. Nat Genet. 1997;16:174. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- 45.Viereck V. Siggelkow H. Tauber S. Raddatz D. Schutze N. Hufner M. Differential regulation of Cbfa1/Runx2 and osteocalcin gene expression by vitamin-D3, dexamethasone, and local growth factors in primary human osteoblasts. J Cell Biochem. 2002;86:348. doi: 10.1002/jcb.10220. [DOI] [PubMed] [Google Scholar]
- 46.Hauschka P.V. Lian J.B. Cole D.E. Gundberg C.M. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 47.Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia. 2011;54:1291. doi: 10.1007/s00125-011-2155-z. [DOI] [PubMed] [Google Scholar]
- 48.Anderson H.C. Reynolds J.J. Pyrophosphate stimulation of calcium uptake into cultured embryonic bones. Fine structure of matrix vesicles and their role in calcification. Dev Biol. 1973;34:211. doi: 10.1016/0012-1606(73)90351-5. [DOI] [PubMed] [Google Scholar]