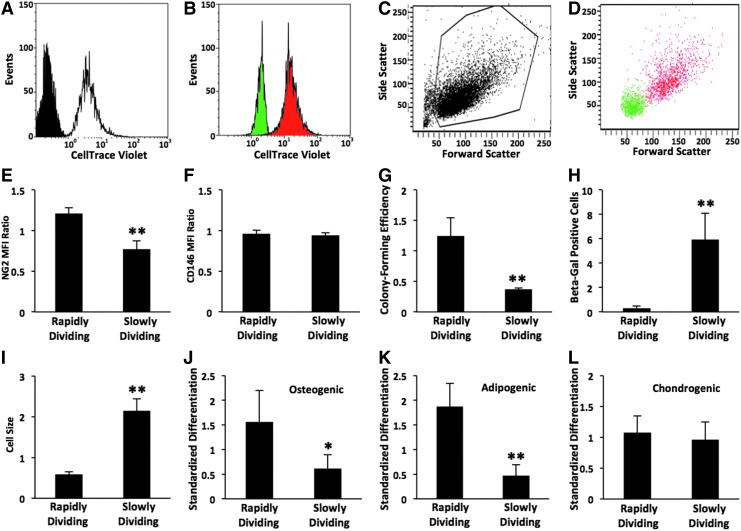
FIG. 5.
Characterization of MSCs sorted into rapidly and slowly dividing populations. Heterogeneous parental MSCs (P2) were labeled with CellTrace Violet and amplified for 4 days. Viable cells with the highest and lowest 10% of fluorescence intensity were sorted by fluorescence-activated cell sorting (FACS) into slowly and rapidly dividing populations, respectively. Representative histograms (A–D) from FACS analysis of 10,000 cells. (A) Parental MSCs labeled with CellTrace Violet (white) are shown relative to unlabeled cells (black). (B) Purity of sorted populations was evaluated by reanalysis of rapidly (green) and slowly (red) dividing cells. Scatter properties of parental MSCs (C, viable gate shown) and sorted populations (D). Surface expression of NG2 (E) and CD146 (F) reported as an MFI ratio as in Figure 1 (n=3–4). (G) Efficiency of sorted cells to form colonies when plated at clonogenic levels (n=4). (H) Senescence-associated β-galactosidase activity as in Figure 4 (n=3). (I) Cell size determined by analysis of 30 images of n=3 subconfluent cultures. Cell potency to exhibit osteo- (J), adipo-, (K) and chondrogenesis (L) as in Supplementary Figure S1 (n=4–5). Threshold values of positive differentiation are given in Supplementary Figure S1. Data are expressed as a mean±standard deviation relative to the sorted parental control with the following phenotype: 240±10 NG2 MFI ratio, 42±6 CD146 MFI ratio, 46%±10% colony-forming efficiency, 9%±2% positive for β-galactosidase, 2900±300 μm2 cell size, and standardized score of 30±7, 170±60, and 8.0±0.8 for osteo-, adipo-, and chondrogenesis, respectively. *p<0.05 and **p<0.01 versus rapidly dividing MSCs.