Abstract
Type I fibrillar collagen is the most abundant protein in the human body, crucial for the formation and strength of bones, skin, and tendon. Proteolytic enzymes are essential for initiation of the assembly of collagen fibrils by cleaving off the propeptides. We report that Mep1a−/− and Mep1b−/− mice revealed lower amounts of mature collagen I compared with WT mice and exhibited significantly reduced collagen deposition in skin, along with markedly decreased tissue tensile strength. While exploring the mechanism of this phenotype, we found that cleavage of full-length human procollagen I heterotrimers by either meprin α or meprin β led to the generation of mature collagen molecules that spontaneously assembled into collagen fibrils. Thus, meprin α and meprin β are unique in their ability to process and release both C- and N-propeptides from type I procollagen in vitro and in vivo and contribute to the integrity of connective tissue in skin, with consequent implications for inherited connective tissue disorders.
Keywords: proteolysis, Ehlers–Danlos syndrome, proteomics, fibrosis, connective tissue
Collagens are the major components of the extracellular matrix (ECM) responsible for tensile strength and stability of connective tissues. Fibril-forming collagens, such as types I, II, and III, are secreted into the ECM as precursor molecules, known as procollagens, that require enzymatic removal of the C- and N-propeptides, an essential step in collagen fibril assembly (1–6). The N-propeptides of procollagen I are cleaved by a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-2, -3, and -14, whereas excision of the C-propeptide is performed by bone morphogenetic protein-1 (BMP-1) and related tolloid-like proteinases (1, 7–11).
As deduced from its known substrates, BMP-1 prefers an aspartic acid residue at the P1′ position (1, 8, 12). Notably, this specificity for negatively charged amino acid residues in the P1′ site is also characteristic of two other members of the astacin family, meprin α and meprin β (13). Meprin proteases are expressed in a range of tissues, where they activate or release growth factors and other biologically active peptides, and are involved in immunological and neuropathological processes, angiogenesis, and matrix remodeling (14–20).
Regulation of ECM remodeling relies on complex molecular interactions and is a key event in many physiological conditions. Defects in the processing of type I procollagen into its mature, fibril-forming form result in such disorders as Ehlers–Danlos syndrome type VII (21). This inherited connective tissue disorder involves mutations that prevent removal of the N-propeptide of type I procollagen. Retention of the amino-terminal propeptides leads to incorporation into the fibril of the partially processed form pN-collagen, resulting in abnormal connective tissue formation (22). A tight balance between synthesis and breakdown of ECM is required for the function of all tissues, and dysregulation leads to pathophysiological events, such as arthritis, cancer, atherosclerosis, aneurysms, and fibrosis (23–34).
We previously reported that both meprin α and meprin β are expressed in human dermal fibroblasts and are overexpressed in skin fibrosis, such as keloids (35). In addition, meprins are known to release C- and N-propeptides from type III procollagen in vitro, where the C-propeptide is cleaved at exactly the same site and even more efficiently than the procollagen C-proteinase (PCP) BMP-1. Here we report that meprin α and meprin β are both C- and N-proteinases for type I procollagen in vitro and in vivo and are important for the maintenance of connective tissue integrity in skin.
Results
Meprin α and Meprin β Release C- and N-Propeptides from Heterotrimeric Human Procollagen I and Miniprocollagen α1(I) Homotrimers in Vitro.
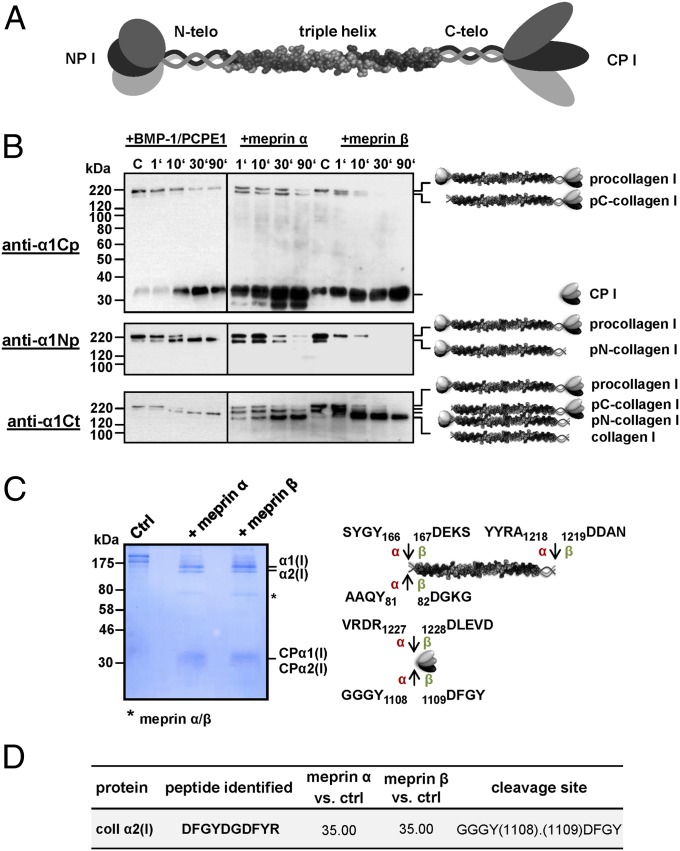
We measured the procollagen proteinase activity of meprin α and meprin β using human recombinant procollagen I heterotrimer as the substrate (Fig. 1A). Procollagen was incubated with meprin α or meprin β, and time-dependent processing was analyzed by Western blotting with specific antibodies raised against procollagen α1(I) C-propeptide, N-propeptide, or C-telopeptide. As a control, procollagen I was also processed with recombinant BMP-1 in the presence of procollagen C-proteinase enhancer (PCPE)-1, which is known to stimulate BMP-1 proteinase activity on fibrillar procollagen (9). As expected, BMP-1 generated pN-collagen by removing the C-propeptides but not the N-propeptides, corresponding to its PCP activity (Fig. 1B). Interestingly, meprin α and meprin β were able to remove both the C-propeptides and the N-propeptides, thereby releasing mature collagen I (Fig. 1B). Clearly, the maturation of procollagen I is more efficient by meprin β compared with meprin α, resulting in full conversion of procollagen after 30 min. Incubation with meprin α not only resulted in release of the full C-propeptides, but also indicated a second cleavage event further C-terminal within the globular propetide. Similarly, this in vitro procollagen C- and N-proteinase activity of meprin α and meprin β could be observed using a miniprocollagen α1(I) homotrimer (Fig. S1).
Fig. 1.
Cleavage of type I procollagen by meprin α and meprin β. (A) Schematic of the type I procollagen molecule consisting of an N-terminal propeptide (NP I), the N-telopeptide (N-telo), the triple helical region, the C-telopeptide (C-telo), and the C-terminal propeptide (CP I). (B) Cleavage of recombinant procollagen heterotrimer by BMP-1, meprin α, and meprin β. Here, 40 nM procollagen I was incubated with each 0.3 nM BMP-1 and PCPE-1 (40 nM), meprin α, or meprin β in a total volume of 50 µL for 1, 10, 30, and 90 min at 37 °C in assay buffer. Subsequently, samples were analyzed by SDS/PAGE (10% wt/vol polyacrylamide) under reducing conditions, followed by Western blotting using anti-collagen α1(I) C-propeptide antibody (anti-α1Cp), anti-collagen α1(I) N-propeptide antibody (anti-α1Np), and anti-collagen α1(I) C-telopeptide antibody (anti-α1Ct). Magic Mark XP (Invitrogen) was used for molecular weight markers. (C) Cleavage of procollagen I for proteomics analysis. Here, 170 μg/mL of recombinant procollagen I heterotrimer was processed with 50 nM meprin α or meprin β in a total volume of 30 µL, then analyzed by SDS/PAGE (10% wt/vol polyacrylamide), followed by staining with Coomassie blue. Arrows indicate cleavage sites analyzed by proteomics for meprin α (α) and meprin β (β). Numbers display positions of amino acids in the full-length protein. (D) C-terminal cleavage site in procollagen α2(I) identified by TAILS. Probability values were calculated with the iProphet algorithm, with high confidence in spectrum-to-peptide assignments. Abundance ratios of meprin α- or meprin β-treated cells vs. untreated control cells (ctrl) >15 identify meprin-generated neo-N-termini as high-confidence cleavage products. Sequences are given in one-letter code; detailed information is available elsewhere (36).
LC-MS–based identification of the cleavage sites in full-length procollagen α1(I) revealed C-terminal cleavage by meprin α and meprin β at position Ala1218/Asp1219 (Fig. 1C). Proteomic analyses of the released C-propeptides revealed cleavage at positions Arg1227/Asp1228 and Tyr1108/Asp1109 in the procollagen α1(I) and α2(I) chains, respectively (Fig. 1C and Fig. S2). Interestingly, the cleavage site at Tyr1108/Asp1109 in procollagen α2(I) was previously identified in cell culture using an MS-based approach, terminal amine isotopic labeling of substrates (TAILS) (Fig. 1D) (36). In addition, N-terminal sequencing of the C-propeptide from homotrimeric miniprocollagen α1(I) released after incubation with meprin β confirmed the cleavage site at position Arg1227/Asp1228 (Fig. S1A). We further identified the meprin α and meprin β cleavage sites in the N-propeptides of the procollagen α1(I) and α2(I) chains at positions Tyr166/Asp167 and Tyr81/Asp82, respectively (Fig. 1C and Fig. S2).
Meprins Trigger Collagen Fibril Formation in Vitro.
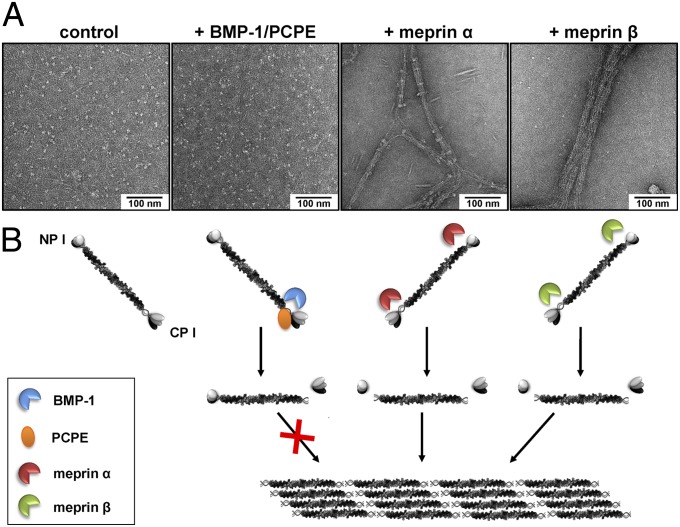
Removal of the globular C- and N-propeptides is the key step in the formation of collagen fibrils. Because meprin α and meprin β show both procollagen C- and N-proteinase activity in vitro, we further investigated whether they were able to trigger the self-assembly of mature collagen into fibrils. By electron microscopy after negative staining, de novo collagen fibril formation could be observed when heterotrimeric full-length procollagen I was incubated with meprin α or meprin β (Fig. 2). After 60 min, incubation with either meprin α or meprin β led to the assembly of fibrils, particularly with meprin β, where the characteristic D-periodicity was apparent (Fig. 2A), consistent with the faster maturation observed by Western blot analysis (Fig. 1B). As expected, BMP-1 was not able to trigger the self-assembly of collagen fibrils; it removed the C-propeptides, but not the N-propeptides, of procollagen I (Figs. 1 and 2 and Fig. S1).
Fig. 2.
De novo fibrillogenesis of type I collagen after cleavage by meprin α or meprin β. (A) Transmission electron micrographs of negatively stained collagen fibrils assembled after cleavage of recombinant procollagen type I heterotrimer by meprin α and meprin β. Here, 100 µg/mL of recombinant procollagen I was incubated in reaction buffer with either 15 nM BMP-1 (plus PCPE-1 equimolar to the substrate), meprin α, or meprin β in a total volume of 10 µL at 37 °C for 60 min. Untreated recombinant procollagen I was visualized as a control. (B) Cartoon summarizing procollagen processing by different proteases and subsequent assembly of collagen fibrils.
Maturation of Type I Procollagen Is Reduced in Skin and Primary Fibroblasts from Mep1a−/− and Mep1b−/− Mice.
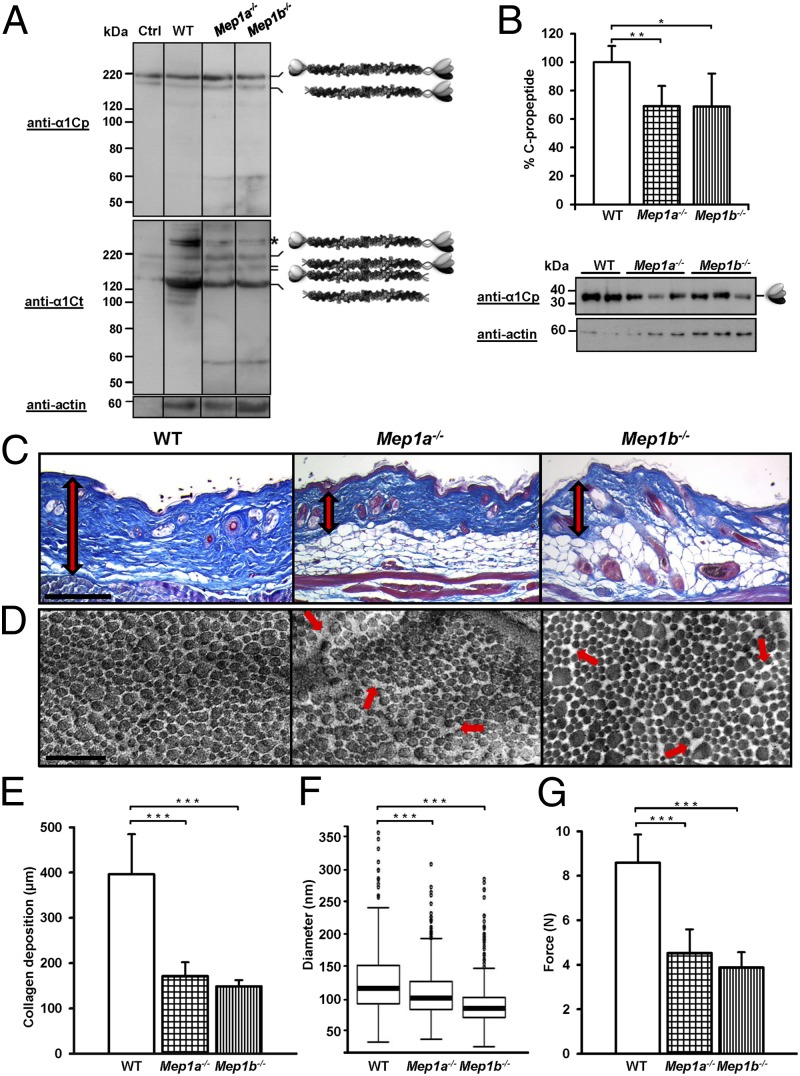
To determine whether meprins are procollagen proteinases in vivo, skin biopsy specimens as well as cultured primary fibroblasts isolated from WT, Mep1a−/−, and Mep1b−/− mice were analyzed by Western blotting using antibodies against the collagen α1(I) C-propeptide or C-telopeptide. Interestingly, procollagen I processing was decreased in the skin of Mep1a−/− and Mep1b−/− mice (Fig. 3 A and B), as well as in the corresponding primary fibroblasts (Fig. S3A). The activity of both procollagen C- and N-proteinase was affected. Extracts of skin from Mep1a−/− and Mep1b−/− mice contained lower amounts of mature collagen I compared with the WT skin (Fig. 3A). In addition, significantly decreased amounts of C-propeptides were detected in Mep1a−/− and Mep1b−/− skin compared with WT, indicating diminished procollagen C-proteinase activity in vivo (Fig. 3B). Increased levels of full-length type I procollagen were detected in primary fibroblasts from meprin KO mice, particularly in meprin α-deficient cells, whereas it was almost completely processed in WT cells (Fig. S3A).
Fig. 3.
In vivo analysis of collagen I maturation, deposition, and mechanical strength in skin of WT, Mep1a−/−, and Mep1b−/− mice. (A) Western blot analyses of skin lysates from WT, Mep1a−/−, and Mep1b−/− mice using antibodies against the collagen α1(I) C-propeptide (anti-α1Cp) or C-telopeptide (anti-α1Ct) with actin as control (anti-actin), demonstrating the proteolytic processing of procollagen I in vivo. The asterisk indicates cross-linked mature collagen I. The control (ctrl) was 40 nM human procollagen I heterotrimer incubated with 0.3 nM meprin α, in a total volume of 50 µL, for 10 min at 37 °C. (B) Western blot analyses and quantification of cleaved C-propeptides in skin lysates from WT, Mep1a−/−, and Mep1b−/− mice using antibodies against the collagen α1(I) C-propeptide (anti-α1Cp) with actin as the control (anti-actin). Western blots from skin lysates from each of five WT, Mep1a−/−, and Mep1b−/− mice were quantified using ImageJ 1.47. *P < 0.05; **P < 0.01. (C) Azan-stained skin cross-sections showing collagen deposition (red arrow) in the dermis of Mep1a−/− and Mep1b−/− mice compared with the skin of age-matched WT mice. (Scale bar: 200 μm.) (D) Dermal collagen fibrils examined by transmission electron microscopy. Fibrils in Mep1a−/− and Mep1b−/− skin cross-sections display a less tightly packed organization (red arrows), whereas the WT collagen fibrils show the characteristic compact and uniform arrangement. (Scale bar: 1 μm.) (E) Dermal collagen deposition quantified by light microscopy. The mean thickness of the various biopsy specimens was obtained by averaging five measurements per section. ***P < 0.001. (F) Boxplot showing the average diameters (in nanometers) for dermal collagen fibrils of WT, Mep1a−/−, and Mep1b−/− mice, obtained by measuring 414 fibrils from three WT mice, 598 fibrils from three Mep1a−/− mice, and 414 fibrils from three Mep1b−/− mice. The 25th percentile is at the bottom of the box, and the 75th percentile is at the top. The median values are shown as horizontal lines, indicating the 50th percentile. Whiskers indicate the lowest datum still within 1.5 interquartile range (IQR) of the lower quartile and the highest datum still within 1.5 IQR of the upper quartile. Outliers are shown as open circles. ***P < 0.001. (G) Measurement of the maximum tensile strength of the skin of Mep1a−/− and Mep1b−/− mice, showing a significant decrease compared with the skin of WT mice. ***P < 0.001.
Lack of Meprin α and Meprin β Leads to Reduced Dermal Collagen Deposition and Impaired Arrangement of Collagen Fibrils.
Owing to the ability of meprins α and β to generate mature type I collagen, as well as the decreased procollagen processing seen in the skin of meprin KO mice, we subsequently analyzed the morphology and deposition of collagen fibrils in situ. Histological examination of dorsal skin from mice lacking meprin α and meprin β exhibited a significantly reduced thickness of the fibrous layer and decreased accumulation of dermal collagen compared with WT mice (Fig. 3 C and E). The dermal collagen layer was ∼170 μm thick in age-matched Mep1a−/− mice and 150 μm thick in Mep1b−/− mice, compared with ∼400 μm in WT mice (Fig. 3E). Remarkably, tissues of Mep1a−/− mice often ruptured during section preparation, providing further evidence of abnormalities in connective tissue formation (Fig. S3B).
To visualize the organization of dermal collagen fibrils, we analyzed skin sections of WT and KO mice by transmission electron microscopy. Interestingly, the arrangement of dermal collagen fibrils in Mep1a−/− and Mep1b−/− mice was impaired compared with that in age-matched WT mice. Whereas collagen fibrils in WT dermis showed a characteristically compact and uniform arrangement, those in Mep1a−/− and Mep1b−/− dermis were often irregularly organized and less tightly packed (Fig. 3D). The average diameter of dermal collagen fibrils was significantly smaller in both Mep1a−/− and Mep1b−/− mice compared with WT mice (Fig. 3F and Fig. S4).
Mep1a−/− and Mep1b−/− Mice Exhibit Reduced Skin Tensile Strength.
Because the deposition and organization of collagen are essential for the integrity of connective tissue, we used a biomechanical approach to quantify the maximum tensile strength of the skin from WT and age-matched meprin KO mice. Remarkably, the skin of mice lacking meprin α or meprin β exhibited significantly reduced maximum tensile strength compared with the skin of WT mice. The dorsal skin of control animals ruptured at ∼8.5 N, whereas the skin of Mep1a−/− and Mep1b−/− mice ripped apart at 4.5 N and 4.0 N, respectively (Fig. 3G). These data correlate with the reduced dermal collagen deposition (Fig. 3E) and the disordered organization of the collagen fibrils as a consequence of the decreased procollagen maturation in the skin of Mep1a−/− and Mep1b−/− mice.
Altered Expression of Genes Associated with Fibrillar Collagen Deposition in Skin of Mep1a−/− and Mep1b−/− Mice.
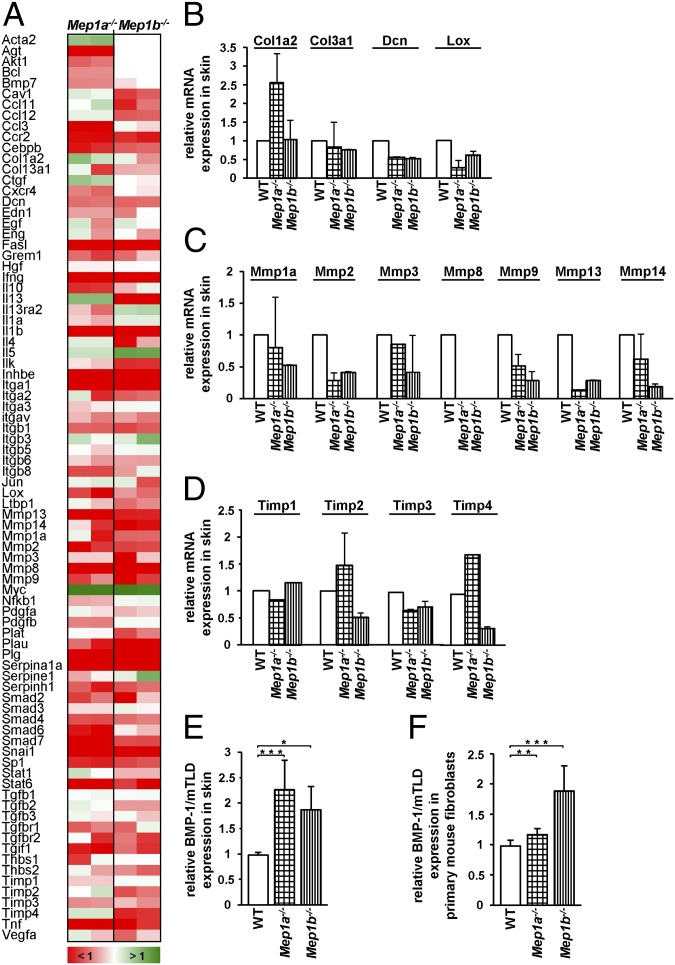
To investigate possible alterations in the expression of genes (84 in total) associated with connective tissue formation, we used a mouse fibrosis PCR array (Fig. 4). Interestingly, expression of Col1a2 was ∼2.5-fold increased in Mep1a−/− mice, but not in Mep1b−/− mice, whereas Col3a1 was slightly decreased in the skin of both Mep1a−/− and Mep1b−/− animals (Fig. 4 A and B). Fibrillar collagen-degrading enzymes, including the interstitial and neutrophil collagenases Mmp1a, 2, 3, 8, 9, 13, and 14, showed a general tendency toward decreased gene expression (Fig. 4 A and C). This was also observed for Timp (tissue inhibitors of metalloproteases) 1 and 3, whereas Timp2 and 4 were significantly increased in Mep1a−/− mice, but not in Mep1b−/− mice (Fig. 4 A and D).
Fig. 4.
Regulation of fibrosis-relevant genes in skin of Mep1a−/− and Mep1b−/− mice. Relative expression of selected genes in the skin of KO and WT mice. (A–D) A total of 84 genes were quantified in duplicate from two different animals of each genotype using a mouse fibrosis PCR array. (A) Color code indicating the extent of change in KO and WT mice (set as 1.0). (B–D) Details of expression of genes relevant for collagen assembly. (E and F) Relative expression of BMP-1/mTLD mRNA in the skin of Mep1a−/− and Mep1b−/− mice (E) and in corresponding primary fibroblasts (F) analyzed by quantitative real-time PCR. n = 3. *P < 0.05; **P < 0.01; ***P < 0.001. Error bars show SD.
Given that PCP activity is associated with the BMP-1/tolloid-like proteinases [i.e., BMP-1 itself, its splice variant mammalian tolloid (mTLD), and mammalian tolloid-like-1 and -2 (mTLL-1 and -2), which were not included in the fibrosis PCR array], we analyzed the expression of these enzymes in the absence of meprin α or meprin β by quantitative real-time PCR. In Mep1a−/− and Mep1b−/− skin, as well as in primary fibroblasts, the mRNA expression of BMP-1/mTLD was significantly increased compared with the WT control (Fig. 4 E and F). In the skin of KO animals, we observed an approximately twofold increase of BMP-1/mTLD expression, which was lower in the Mep1a−/− fibroblasts but still significant. Interestingly, in the skin of Mep1a−/− and Mep1b−/− mice, the mRNA expression of mTLL-1 was down-regulated to ∼25% compared with WT, whereas it was unchanged in Mep1a−/− primary fibroblasts and down-regulated to 2% in Mep1b−/− primary fibroblasts (Fig. S5 A and B). In contrast, mTLL-2 expression was increased more than twofold in Mep1a−/− and Mep1b−/− fibroblasts (Fig. 5C); however, mTLL-2 mRNA was not detectable in samples of murine skin.
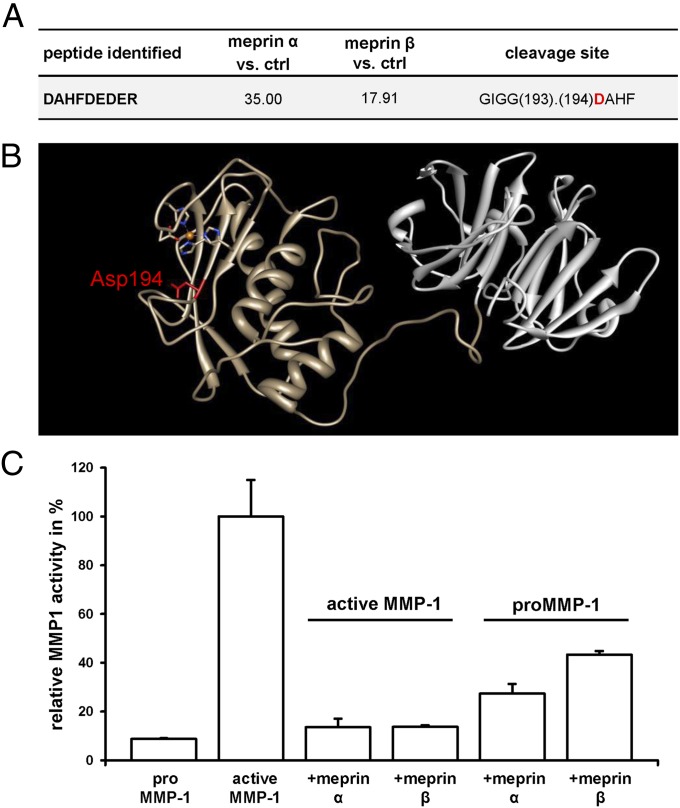
Fig. 5.
Influence of meprin α and meprin β on the collagenolytic activity of MMP-1. (A) Meprin cleavage site in MMP-1 identified by TAILS. Probability values were calculated using the iProphet algorithm, with high confidence in spectrum to peptide assignments. Abundance ratios of meprin α- or meprin β-treated cells vs. control cells (ctrl) >15 identify meprin-generated neo-N-termini as high-confidence cleavage products. Sequences are given in one-letter code; detailed information is available elsewhere (36). (B) Crystal structure of MMP-1 (Protein Data Bank ID code 2CLT), with the meprin cleavage site (Asp194 in P1′) identified by TAILS highlighted in red. The catalytic domain of MMP-1 is in brown, and the hemopexin domain is in gray. The catalytic zinc in the active site cleft is in orange, complexed by three histidines (blue) and one aspartate residue (red). The image was produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California San Francisco (53). (C) Relative activity of MMP-1 with or without meprin α or meprin β was determined using the fluorogenic peptide MOCAc-Pro-Leu-Gly-Leu-A2(Dnp)-Ala-Arg-NH2. Proteolytic activity was calculated relative to the emission at 405 nm with an excitation at 320 nm. When proMMP-1 was first processed with meprin α or meprin β and subsequently activated with APMA, the collagenase activity decreased to 27% and 43%, respectively, of that in controls. When MMP-1 was first activated with APMA and further processed with meprin α or meprin β, remaining collagenolytic activity was 13%.
Meprins Cleave Matrix Metalloproteinase 1 and Reduce Its Collagenolytic Activity in Vitro.
Matrix metalloproteinases (MMPs) play a key role in ECM remodeling and exhibit proteolytic activity against mature type I collagens (37). In the MS-based approach TAILS, which enables the identification of native protein substrates in cellulo, MMP-1 was identified as a substrate for meprin α and meprin β revealing a cleavage site within the catalytic domain (Fig. 5 A and B). Because an intact active site is essential for the catalytic activity of MMP-1, we analyzed whether cleavage by meprins directly affects MMP-1 collagenolytic activity. MMP-1 is secreted as a zymogen and requires the removal of its propeptide (38). The prodomain was displaced from the active site by treatment with p-aminophenylmercuric acetate (APMA). We investigated differences in the collagenase activity of MMP-1 cleaved by meprin α and meprin β compared with the unprocessed enzyme by activity assays (Fig. 5C). When proMMP-1 was first incubated with meprin α or meprin β and subsequently activated with APMA, the collagenase activity decreased to 27% and 43%, respectively, of that of untreated active MMP-1. Even lower activity of approximately 13% was measured when active MMP-1 was processed by meprin α or meprin β (Fig. 5C).
Discussion
The integrity and stability of connective tissue is regulated mainly through a balance between collagen assembly and degradation (3). The most abundant fibrillar collagens, types I, II, and III, are synthesized as procollagens with globular C- and N-terminal propeptides that impede the assembly of the collagen fibril and must be removed by procollagen C- and N-proteinases (PCP and PNP, respectively) (2). It has been demonstrated that BMP-1/tolloid-like proteinases provide PCP activity in vitro and in vivo (2). Recently it became clear that astacins, including meprin α, meprin β, and BMP-1/tolloid-like proteinases, exhibit a unique family-wide cleavage specificity with negatively charged amino acid residues in P1′ (13). In this regard, it was demonstrated that both meprins process the C-propeptide of type III procollagen in vitro at exactly the same site but even more efficiently than BMP-1 (35).
In the present study, we report on the physiological relevance of the unique ability of meprin α and meprin β to remove the both the C- and N-propeptides of type I procollagen, subsequently releasing fibril-forming mature collagen molecules. The carboxyl-terminal cleavage sites in the proα1(I) chain generated by both meprins were identified as Ala1218/Asp1219, identical to the BMP-1 cleavage site, and also Arg1227/Asp1228, nine residues C-terminal to the BMP-1 cleavage site (Fig. S6) (39). In addition, we found that meprins cleave the proα2(I) chain at Tyr1108/Asp1109, this time 11 residues N-terminal to the known BMP-1 site. In this connection, we note that evidence of two N-terminal sequences for the C-propeptides of procollagen II (chondrocalcin) was previously reported by van der Rest et al. (40), one corresponding to the classical BMP-1 site and the other being 10 residues toward the C-terminus and corresponding to the meprin cleavage site seen here in the proα1(I) chain. In summary, these cleavages result in the release of proα1 and proα2 C-propeptides, which is a crucial step in collagen assembly, as demonstrated by our de novo fibril formation experiments.
Pappano et al. (41) reported residual PCP activity using mouse embryonic fibroblasts (MEFs) isolated from Bmp1−/−/Tll1−/− mice. This indicates that other proteases contribute to the maturation of procollagen. Whereas Pappano et al. (41) suggested that mTLL-2 might fulfill this role, here we provide evidence that meprins may be responsible as well. Interestingly, the collagen fibrils in BMP-1–deficient embryos are relatively small in diameter and have a less-organized arrangement compared with WT controls (42), which is similar to the phenotype seen in Mep1a−/− and Mep1b−/− mice. Along with the phenotype of Mep1a−/− and Mep1b−/− mice at the histological level, the observed reduced levels of procollagen I conversion in skin and primary fibroblasts isolated from meprin KO mice provide further evidence that meprin α and meprin β exhibit PCP activity in vivo. Interestingly, significantly fewer Mep1b−/− mice (∼50%) are born compared with WT mice, indicating essential functions of this protease already during early embryonic development (43). Moreover, morpholino-induced meprin knockdown in zebrafish embryos revealed severe defects in organogenesis and tail morphology, which also might be linked to reduced collagen fibril assembly in these animals (15).
Most interestingly, both meprins cleave off the N-propeptides of type I procollagen in vitro, as demonstrated by immunoblotting of recombinant procollagen I. Identification of meprin cleavage sites by MS revealed positions Tyr166/Asp167 in the α1 chain and Tyr81/Asp82 in the α2 chain within an amino acid region known to cause Ehlers–Danlos syndrome VIIB when deleted by impaired splicing (see below). Previous studies demonstrated the requirement of ADAMTS-2 in the removal of the N-propeptide of type I collagen. However, analogous to the PCP activity of BMP-1/tolloid-like proteinases, residual PNP activity was detected in ADAMTS-2 KO mice (7). It has been postulated that ADAMTS-14 may perform this activity, but this has not yet been demonstrated in vivo. Although N-terminal sequencing of mature type I collagen from rat skin or tendon revealed the ADAMTS-2 cleavage site (44), different truncated forms likely are present in vivo. The dominant forms of cross-linked α1(I) N-telopeptides identified by MS in bone and urine revealed the sequences YGYDEKSTGGIS and DEKSTGG, respectively (45). The latter peptide corresponds to the dominant meprin cleavage site in procollagen I, which occurs six residues C-terminal to the ADAMTS-2 site (Fig. 1C and Figs. S2B and S6). We also note that the meprin cleavage site in the proα2(I) chain is immediately C-terminal to the ADAMTS-2 site (Fig. S6). Furthermore, for fibrillar collagen V, the N terminus was shown to be diversely processed in vivo (46). Taken together, these various observations on the C- and N-terminal procollagen cleavage sites suggest greater heterogeneity than was originally thought, which now can be accounted for by the action of meprins.
Retention of the N-propeptide results in a distinctive phenotype in vivo, as described for the Ehlers–Danlos syndrome type VII (EDS VII) (47, 48). EDS VII is an inherited autosomal disorder characterized either by an impaired splicing of exon 6, leading to the loss of the N-proteinase cleavage site and surrounding residues in the Col1A1 or Col1A2 genes (49), or by deficient ADAMTS-2 activity (50, 51). These mutations result in reduced N-terminal processing of procollagen I and subsequent disordered collagen assembly. In skin biopsy specimens from patients with EDS VII, collagen fibrils are more loosely and randomly organized and have smaller diameters compared with normal dermal collagen fibrils, similar to the phenotype observed here in Mep1a−/− and Mep1b−/− mice (52). The reduced PNP activity in skin and fibroblasts of meprin KO mice, as well as the distinct phenotype, provide strong evidence that the enzymatic activity of meprins is physiologically relevant for the removal of the N-propeptide in vivo.
Although BMP-1 and mTld are overexpressed in meprin KO mice and may partially compensate for the loss of PCP activity, whereas collagenases are decreased in meprin-deficient animals, this is apparently insufficient to maintain the full conversion of procollagen into the physiologically required amounts of fibril-forming molecules. The skin phenotype of Mep1a−/− and Mep1b−/− mice is also consistent with the ability of both meprins to process MMP-1 within the catalytic domain, consequently disarming the collagenolytic activity of MMP-1 in vitro. Thus, MMP-1 activity might be increased in the absence of meprin α or meprin β, resulting in further decreased collagen deposition.
Taken together, our data demonstrate that meprins are physiologically relevant procollagen proteinases. They are required for the assembly of collagen in skin and maintenance of the integrity of the connective tissue. The likely association of meprins with inherited collagen disorders and keloids/hypertrophic scars makes them promising candidates for targeted therapeutic applications to limit fibrosis or related diseases.
Materials and Methods
Recombinant proteins were expressed in insect cells (meprin α and meprin β), HEK 293-EBNA cells (BMP-1 and PCPE-1), CHO cells (MMP-1), or yeast (procollagen I). Cleavage sites for meprin α and meprin β in human procollagen I heterotrimers were identified by MS. A de novo fibril formation assay using procollagen I heterotrimers was visualized by transmission electron microscopy. Skin of 12- to 16-wk-old WT, Mep1a−/−, and Mep1b−/− mice was used to analyze collagen processing, deposition, and fibril organization, as well as the tensile strength in vivo.
Analysis of connective tissue-associated genes was done using a mouse fibrosis PCR array (SA Biosciences). ΔΔCp values were used to calculate the relative expression for each data point. The activity of MMP-1 in the presence and absence of meprin α and meprin β was determined using the quenched fluorogenic peptide MOCAc-Pro-Leu-Gly-Leu-A2(Dnp)-Ala-Arg-NH2 (R&D Systems). Data are presented as mean ± SD. Detailed information on study methods is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Larry W. Fisher for the excellent collagen I antibodies, James W. Polarek for the Pichia pastoris cells overexpressing recombinant human procollagen I, Jürgen Markl for scientific support, and Dominique Mazzocut for N-terminal Edman sequencing. This work was supported by Deutsche Forschungsgemeinschaft Grant BE 4086/1-2 and Collaborative Research Center (CRC877) Projects A9 (C.B.-P.) and Z2 (A.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305464110/-/DCSupplemental.
References
- 1.Moali C, Hulmes DJS. Extracellular Matrix: Pathobiology and Signaling. In: Karamanos M, editor. Roles and Regulation of BMP-1/Tolloid-Like Proteinases: Collagen/Matrix Assembly, Growth Factor Activation and Beyond. Berlin: De Gruyter; 2012. [Google Scholar]
- 2.Hulmes DJS. Collagen diversity, synthesis and assembly. In: Fratzl P, editor. Collagen: Structure and Mechanics. New York: Springer; 2008. pp. 15–74. [Google Scholar]
- 3.Hulmes DJ. Building collagen molecules, fibrils, and suprafibrillar structures. J Struct Biol. 2002;137(1-2):2–10. doi: 10.1006/jsbi.2002.4450. [DOI] [PubMed] [Google Scholar]
- 4.Kadler KE, Holmes DF, Graham H, Starborg T. Tip-mediated fusion involving unipolar collagen fibrils accounts for rapid fibril elongation, the occurrence of fibrillar branched networks in skin and the paucity of collagen fibril ends in vertebrates. Matrix Biol. 2000;19(4):359–365. doi: 10.1016/s0945-053x(00)00082-2. [DOI] [PubMed] [Google Scholar]
- 5.Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118(Pt 7):1341–1353. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- 6.Widmer C, et al. Molecular basis for the action of the collagen-specific chaperone Hsp47/SERPINH1 and its structure-specific client recognition. Proc Natl Acad Sci USA. 2012;109(33):13243–13247. doi: 10.1073/pnas.1208072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colige A, et al. Cloning and characterization of ADAMTS-14, a novel ADAMTS displaying high homology with ADAMTS-2 and ADAMTS-3. J Biol Chem. 2002;277(8):5756–5766. doi: 10.1074/jbc.M105601200. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins DR, Keles S, Greenspan DS. The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol. 2007;26(7):508–523. doi: 10.1016/j.matbio.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moali C, et al. Substrate-specific modulation of a multisubstrate proteinase: C-terminal processing of fibrillar procollagens is the only BMP-1-dependent activity to be enhanced by PCPE-1. J Biol Chem. 2005;280(25):24188–24194. doi: 10.1074/jbc.M501486200. [DOI] [PubMed] [Google Scholar]
- 10.Bourhis JM, et al. Structural basis of fibrillar collagen trimerization and related genetic disorders. Nat Struct Mol Biol. 2012;19(10):1031–1036. doi: 10.1038/nsmb.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarras MP., Jr BMP-1 and the astacin family of metalloproteinases: A potential link between the extracellular matrix, growth factors and pattern formation. Bioessays. 1996;18(6):439–442. doi: 10.1002/bies.950180604. [DOI] [PubMed] [Google Scholar]
- 12.Schechter I. Mapping of the active site of proteases in the 1960s and rational design of inhibitors/drugs in the 1990s. Curr Protein Pept Sci. 2005;6(6):501–512. doi: 10.2174/138920305774933286. [DOI] [PubMed] [Google Scholar]
- 13.Becker-Pauly C, et al. Proteomic analyses reveal an acidic prime side specificity for the astacin metalloprotease family reflected by physiological substrates. Mol Cell Proteomics. 2011;10(9):009233. doi: 10.1074/mcp.M111.009233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vazeille E, et al. Role of meprins to protect ileal mucosa of Crohn’s disease patients from colonization by adherent-invasive E. coli. PLoS ONE. 2011;6(6):e21199. doi: 10.1371/journal.pone.0021199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schütte A, Hedrich J, Stöcker W, Becker-Pauly C. Let it flow: Morpholino knockdown in zebrafish embryos reveals a pro-angiogenic effect of the metalloprotease meprin alpha2. PLoS ONE. 2010;5(1):e8835. doi: 10.1371/journal.pone.0008835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banerjee S, et al. Balance of meprin A and B in mice affects the progression of experimental inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2011;300(2):G273–G282. doi: 10.1152/ajpgi.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herzog C, et al. Meprin A and meprin alpha generate biologically functional IL-1beta from pro-IL-1beta. Biochem Biophys Res Commun. 2009;379(4):904–908. doi: 10.1016/j.bbrc.2008.12.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruse MN, et al. Human meprin alpha and beta homo-oligomers: Cleavage of basement membrane proteins and sensitivity to metalloprotease inhibitors. Biochem J. 2004;378(Pt 2):383–389. doi: 10.1042/BJ20031163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jefferson T, et al. Metalloprotease meprin beta generates nontoxic N-terminal amyloid precursor protein fragments in vivo. J Biol Chem. 2011;286(31):27741–27750. doi: 10.1074/jbc.M111.252718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broder C, Becker-Pauly C. The metalloproteases meprin α and meprin β: Unique enzymes in inflammation, neurodegeneration, cancer and fibrosis. Biochem J. 2013;450(2):253–264. doi: 10.1042/BJ20121751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ. Ehlers–Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK) Ehlers–Danlos syndromes: Revised nosology, Villefranche, 1997. Am J Med Genet. 1998;77(1):31–37. doi: 10.1002/(sici)1096-8628(19980428)77:1<31::aid-ajmg8>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 22.Lenaers A, Ansay M, Nusgens BV, Lapière CM. Collagen made of extended chains, procollagen, in genetically-defective dermatosparaxic calves. Eur J Biochem. 1971;23(3):533–543. doi: 10.1111/j.1432-1033.1971.tb01651.x. [DOI] [PubMed] [Google Scholar]
- 23.Spinale FG. Matrix metalloproteinases: Regulation and dysregulation in the failing heart. Circ Res. 2002;90(5):520–530. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- 24.Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85(1):1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 25.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: A tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3(3):207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 26.Kadoglou NP, Liapis CD. Matrix metalloproteinases: Contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysms. Curr Med Res Opin. 2004;20(4):419–432. doi: 10.1185/030079904125003143. [DOI] [PubMed] [Google Scholar]
- 27.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69(3):562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Nagase H, Brew K. Engineering of tissue inhibitor of metalloproteinases mutants as potential therapeutics. Arthritis Res. 2002;4(Suppl 3):S51–S61. doi: 10.1186/ar573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LeBleu VS, et al. Identification of human epididymis protein-4 as a fibroblast-derived mediator of fibrosis. Nat Med. 2013;19(2):227–231. doi: 10.1038/nm.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iyer S, Visse R, Nagase H, Acharya KR. Crystal structure of an active form of human MMP-1. J Mol Biol. 2006;362(1):78–88. doi: 10.1016/j.jmb.2006.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tam EM, Moore TR, Butler GS, Overall CM. Characterization of the distinct collagen binding, helicase and cleavage mechanisms of matrix metalloproteinase 2 and 14 (gelatinase A and MT1-MMP): The differential roles of the MMP hemopexin c domains and the MMP-2 fibronectin type II modules in collagen triple helicase activities. J Biol Chem. 2004;279(41):43336–43344. doi: 10.1074/jbc.M407186200. [DOI] [PubMed] [Google Scholar]
- 33.Manka SW, et al. Structural insights into triple-helical collagen cleavage by matrix metalloproteinase 1. Proc Natl Acad Sci USA. 2012;109(31):12461–12466. doi: 10.1073/pnas.1204991109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckhard U, Schönauer E, Nüss D, Brandstetter H. Structure of collagenase G reveals a chew-and-digest mechanism of bacterial collagenolysis. Nat Struct Mol Biol. 2011;18(10):1109–1114. doi: 10.1038/nsmb.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kronenberg D, et al. Processing of procollagen III by meprins: New players in extracellular matrix assembly? J Invest Dermatol. 2010;130(12):2727–2735. doi: 10.1038/jid.2010.202. [DOI] [PubMed] [Google Scholar]
- 36.Jefferson T, et al. The substrate degradome of meprin metalloproteases reveals an unexpected proteolytic link between meprin β and ADAM10. Cell Mol Life Sci. 2013;70(2):309–333. doi: 10.1007/s00018-012-1106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Wart HE, Birkedal-Hansen H. The cysteine switch: A principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA. 1990;87(14):5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS. Bone morphogenetic protein-1: The type I procollagen C-proteinase. Science. 1996;271(5247):360–362. doi: 10.1126/science.271.5247.360. [DOI] [PubMed] [Google Scholar]
- 40.Van der Rest M, Rosenberg LC, Olsen BR, Poole AR. Chondrocalcin is identical with the C-propeptide of type II procollagen. Biochem J. 1986;237(3):923–925. doi: 10.1042/bj2370923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pappano WN, Steiglitz BM, Scott IC, Keene DR, Greenspan DS. Use of Bmp1/Tll1 doubly homozygous null mice and proteomics to identify and validate in vivo substrates of bone morphogenetic protein 1/tolloid-like metalloproteinases. Mol Cell Biol. 2003;23(13):4428–4438. doi: 10.1128/MCB.23.13.4428-4438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki N, et al. Failure of ventral body wall closure in mouse embryos lacking a procollagen C-proteinase encoded by Bmp1, a mammalian gene related to Drosophila tolloid. Development. 1996;122(11):3587–3595. doi: 10.1242/dev.122.11.3587. [DOI] [PubMed] [Google Scholar]
- 43.Norman LP, Jiang W, Han X, Saunders TL, Bond JS. Targeted disruption of the meprin beta gene in mice leads to underrepresentation of knockout mice and changes in renal gene expression profiles. Mol Cell Biol. 2003;23(4):1221–1230. doi: 10.1128/MCB.23.4.1221-1230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang AH, Bornstein P, Piez KA. The amino acid sequence of peptides from the cross-linking region of rat skin collagen. Biochemistry. 1967;6(3):788–795. doi: 10.1021/bi00855a019. [DOI] [PubMed] [Google Scholar]
- 45.Hanson DA, et al. A specific immunoassay for monitoring human bone resorption: Quantitation of type I collagen cross-linked N-telopeptides in urine. J Bone Miner Res. 1992;7(11):1251–1258. doi: 10.1002/jbmr.5650071119. [DOI] [PubMed] [Google Scholar]
- 46.Moradi-Améli M, et al. Diversity in the processing events at the N-terminus of type-V collagen. Eur J Biochem. 1994;221(3):987–995. doi: 10.1111/j.1432-1033.1994.tb18815.x. [DOI] [PubMed] [Google Scholar]
- 47.Pope FM, Burrows NP. Ehlers–Danlos syndrome has varied molecular mechanisms. J Med Genet. 1997;34(5):400–410. doi: 10.1136/jmg.34.5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hulmes DJ, et al. Pleomorphism in type I collagen fibrils produced by persistence of the procollagen N-propeptide. J Mol Biol. 1989;210(2):337–345. doi: 10.1016/0022-2836(89)90335-5. [DOI] [PubMed] [Google Scholar]
- 49.Watson RB, et al. Ehlers–Danlos syndrome type VIIB: Incomplete cleavage of abnormal type I procollagen by N-proteinase in vitro results in the formation of copolymers of collagen and partially cleaved pNcollagen that are near circular in cross-section. J Biol Chem. 1992;267(13):9093–9100. [PubMed] [Google Scholar]
- 50.Colige A, et al. Novel types of mutation responsible for the dermatosparactic type of Ehlers–Danlos syndrome (type VIIC) and common polymorphisms in the ADAMTS2 gene. J Invest Dermatol. 2004;123(4):656–663. doi: 10.1111/j.0022-202X.2004.23406.x. [DOI] [PubMed] [Google Scholar]
- 51.Holmes DF, Watson RB, Steinmann B, Kadler KE. Ehlers–Danlos syndrome type VIIB: Morphology of type I collagen fibrils formed in vivo and in vitro is determined by the conformation of the retained N-propeptide. J Biol Chem. 1993;268(21):15758–15765. [PubMed] [Google Scholar]
- 52.Giunta C, Chambaz C, Pedemonte M, Scapolan S, Steinmann B. The arthrochalasia type of Ehlers–Danlos syndrome (EDS VIIA and VIIB): The diagnostic value of collagen fibril ultrastructure. Am J Med Genet A. 2008;146A(10):1341–1346. doi: 10.1002/ajmg.a.32213. [DOI] [PubMed] [Google Scholar]
- 53.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.