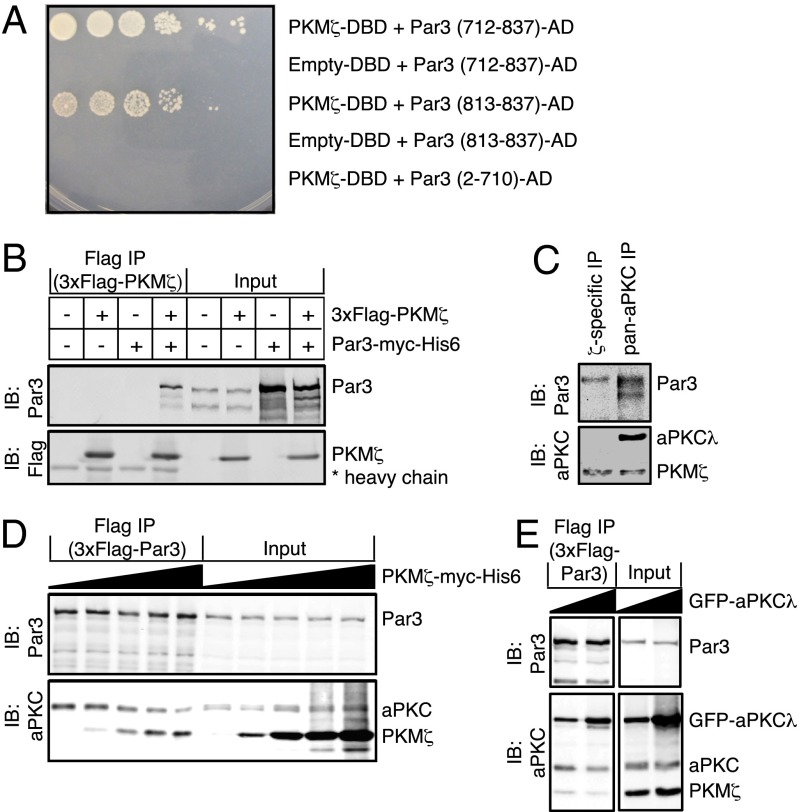
Fig. 4.
PKM-ζ and aPKC-λ mutually compete for biochemical interaction with Par3. (A) Yeast two-hybrid interaction of PKM-ζ and the indicated amino acid sequences of Par3. DBD, DNA-binding domain; AD, activation domain. (B) 3xFlag–PKM-ζ alone, Par3–myc–His6 alone, or both were expressed in HEK293 cells as indicated (+). Anti-Flag antibody was used for immunoprecipitation. Immunoprecipitates and input samples were resolved by SDS/PAGE and immunoblotted with antibodies against Par3 and Flag. (C) ζ-Specific or pan-aPKC antibodies were used for immunoprecipitation from E19 rat hippocampal lysates. Immunoprecipitates were resolved by SDS/PAGE and immunoblotted with antibodies against Par3 and pan-aPKC. (D) Stably transfected Flag-tagged Par3 HEK293 cells were transfected with increasing concentrations of a PKM-ζ–myc–His6-encoding plasmid. Anti-Flag antibody was used for immunoprecipitation. Immunoprecipitates and input samples were resolved by SDS/PAGE and immunoblotted with antibodies against Par3 and pan-aPKC. (E) Stably transfected Flag-tagged Par3 HEK293 cells were transfected with PKM-ζ–myc–His6 and increasing concentrations of GFP–aPKC-λ–encoding plasmid. Anti-Flag antibody was used for immunoprecipitation. Immunoprecipitates and input samples were resolved by SDS/PAGE and immunoblotted with antibodies against Par3 and pan-aPKC. Data are representative images of three independent experiments.