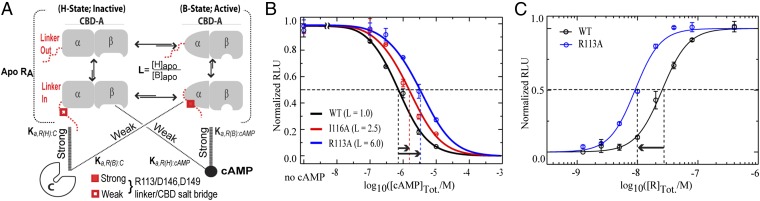
Fig. 4.
Linker controls both inhibition and activation of PKA by modulating the B vs. H equilibrium of apo CBD-A. (A) Thermodynamic model of cAMP-dependent activation of PKA. The linker (red) is dynamic and samples conformations in which it is fully solvent exposed (“linker out” and conformations in which it transiently approaches the well-folded CBD-A (“linker in”), preferentially interacting with the B state of CBD-A (gray) and shifting the overall H vs. B apo equilibrium toward its midpoint (i.e., L = [H]apo/[B]apo ∼ 1). The H and B conformations exhibit high affinity for C and cAMP, respectively (thick lines), but weaker cross-interactions occur (thin dotted lines) (Table S3). The α- and β-subdomains of CBD-A are shown schematically in both the inactive (H) and active (B) conformations. (B) PKA activation by cAMP for wt RIα (91-244) (black circles) and the I116A (red circles) and R113A (blue circles) mutants, which weaken the B-selective interaction of the linker with CBD-A. Data were fit by activation curves (solid lines) computed based on the thermodynamic model in A (SI Text). (C) PKA inhibition by wt (black) and R113A (blue) RIα (91-244).