Fig. 5.
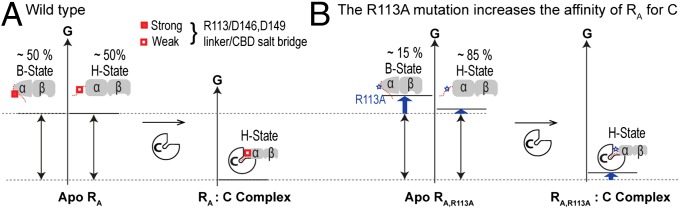
Effect of the R113A RA linker mutation on the binding free energy of the RA:C kinase-inhibitory complex. (A) Free-energy changes occurring upon formation of the wt RA:C complex. The C subunit selects primarily for the H state of apo wt RA. (B) As in A but for the R113A RA:C complex. In apo R113A RA, the H state is more populated than in apo wt RA, because R113 forms stable salt bridges in the B- but not in the H state of apo wt RA. For clarity, the weak cross-interactions between C and the B state of apo RA are not shown.
