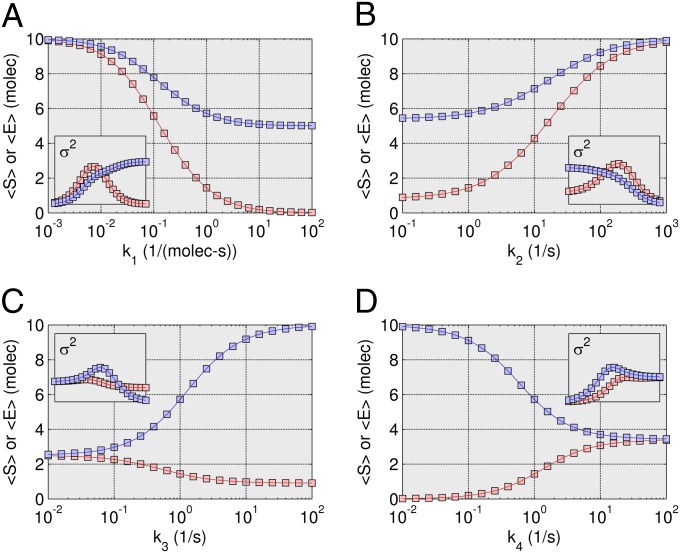
Fig. 3.
Steady-state results for Michaelis–Menten model. The steady-state results for a wide range of kinetic parameter values [centered around k1 = 1 (1/molecules-s), k2 = 1 (1/s), k3 = 1 (1/s), and k4 = 1 (1/s)] for the Michaelis–Menten model (S0 = 10; E0 = 10). (A) Both the mean substrate (S, red) and enzyme (E, blue) count are shown for fourth-order ZI closure (solid lines) and compared with SSA simulations (squares) with one million trajectories. (B–D) Identical conditions as in A except for k2, k3, and k4, respectively. Note that as each parameter is ranged in A–D the rest are held constant. Insets show variances for S (line for ZI closure, circle for SSA), and E (line for ZI closure, square for SSA).