Abstract
Invasive pneumococcal disease (IPD) is a potential life-threatening situation that requires immediate recognition and treatment. Cerebrovascular complications are uncommon and have been reported less frequently in adults than in children. We report a case of 59-year-old man with IPD complicated by cerebral vasculitis, transient central diabetes insipidus and spondylodiscitis. Each of these complications is rare and needs specific approach. Their association is even rarer and to the best of our knowledge this is the first case reported.
Background
Invasive pneumococcal disease (IPD) is defined as a proven isolation of Streptococcus pneumoniae bacteria from normally sterile sites such as blood or cerebrospinal fluid. It remains a major cause of morbidity and mortality worldwide despite the availability of antibiotic therapy and vaccines.1–5 Host as well as bacterial factors contribute to IPD pathogenicity. Ethnicity, extremes of age, comorbidities and alcoholism are well-known host risk factors associated with increased susceptibility and higher mortality.3 6
Pneumococcal meningitis remains a potentially devastating disease with high mortality rate and neurological damage among those who survive.1 2 7 8 Focal neurological findings may be present during the acute phase of bacterial meningitis, but more often occur after a few days as immunological complication of meningitis. Central diabetes insipidus (CDI) is rarely associated with bacterial meningitis and has been reported mainly in paediatric patients.9–11 Neither the benefits of dexamethasone use nor its withdrawal are clear in the literature. Although one case of induced CDI by IPD has been reported, to our knowledge, this is the first case in the adult population of IPD complicated by cerebral vasculitis, transient CDI and spondylodiscitis.
Case presentation
We report a case of a 59-year-old man, an alcoholic (240 g/day), admitted with fever (39.3°C), vomiting and blurred mental state. He was stuporous with nuchal rigidity and positive Kernig sign. The lumbar puncture revealed a purulent cerebral spinal fluid (CSF). Urinalysis and chest radiograph were normal and HIV test was negative. Empirical intravenous cefotaxime, ampiciline and adjunctive dexamethasone were initiated.
On day 3, penicillin-sensitive S pneumoniae was identified in the CSF and blood cultures. At this time, ampiciline was discontinued and dexamethasone suspended on the day 4. Meanwhile, on day 8, the patient developed severe headache, blurred vision and generalised seizures. A second brain CT scan did not reveal additional changes and repeated lumbar puncture showed decreasing inflammatory parameters and sterile CSF culture. Even though antibiotic therapy was modified to vancomycin to treat potentially resistant pneumococci, 3 days later our patient developed left hemiplegia and became more stuporous. MRI showed numerous small lesions in the white matter and basal ganglia (figure 1) highly evocative of cerebral arteritis in the perforating branches of the middle cerebral artery. The EEG showed focal disturbances in the right frontotemporal area and background slow activity. The immunological panel was negative. Therefore, due to the unfavourable outcome, steroids were restarted as adjunctive therapy (methylprednisolone, 500 mg daily for 3 days followed by prednisolone 1 mg/kg/day).
Figure 1.
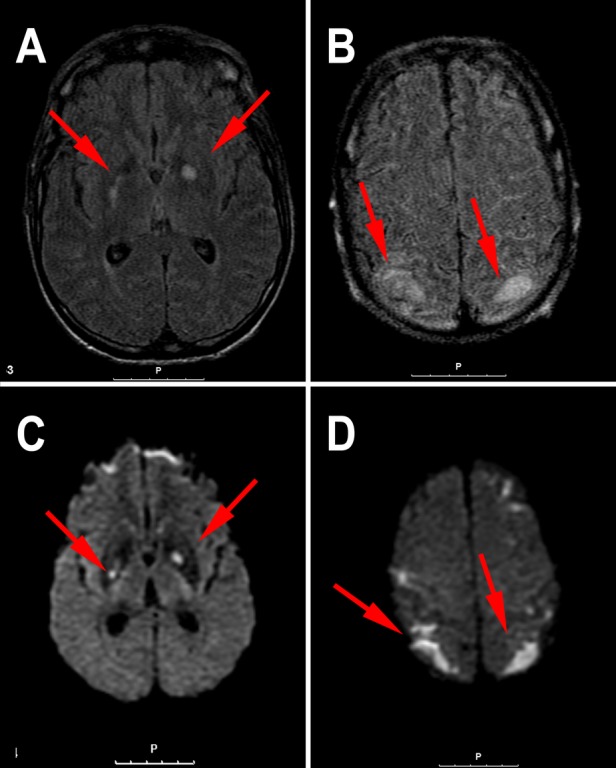
An MRI scan of the patient's brain. Axial fluid-attenuated inversion recovery (A and B) showing multiple hyperintense foci namely in the basal ganglia, right posterior arm of the internal capsule, brain stem (not shown in these images), corticosubcortical frontal and parietal lobes. Axial diffusion weighted imaging (C and D) these lesions show up with strong hypersinal suggesting several areas of acute ischaemic lesions (cerebral vasculitis).
Parallel to the neurological involvement, on day 5 of admission, the patient developed polyuria (maximum daily urine output of 14 L on day 9), serum sodium level increased from 134 to 167 mmol/L and serum osmolality from 273 to 344 mmol/kg H2O. Desmopressin was started at the dose of 2 µg intravenous and the urine output, sodium and osmolality returned to normal, suggesting CDI (table 1). Owing to non-adherence to the intranasal route subcutaneous desmopressin was maintained and withdrawn in the following weeks according to urine output.
Table 1.
Laboratory values for sodium and osmolality
| Baseline (D4) | D9 (during 12 h) | After arginine vasopressin | |
|---|---|---|---|
| Serum sodium (mmol/L; normal range 135–145) | 134 | 157 | 139 |
| Osmolality (mmol/kg H2O) | |||
| Serum (normal range: 275–295) | 273 | 344 | 272 |
| Urine (normal range: 50–1200) | 474 | 110 | 476 |
On day 25, he was out of coma, out of steroids and the antibiotic therapy was suspended. Three days later, new malaise, fever with rigors started with prostration and low back pain that made him impossible to mobilise. The physical examination was normal except for pain elicited by pressure on the L3–L4 and serum inflammatory markers reappeared, hence spondylodiscitis was considered. The plain radiograph of lumbar spine showed degenerative changes at multiples levels. Yet, pursuing our hypothesis, on day 32, a MRI was performed and demonstrated decreased signal intensity on the T1-weighted image and an increased intensity on the T2-weighted image in the anteroinferior aspect of the body of L3–L4 consistent with spondylodiscitis (figure 2). Antibiotic therapy with vancomycin was restarted and meropenem was added considering nosocomial enterobacteriaceae infection risk.
Figure 2.
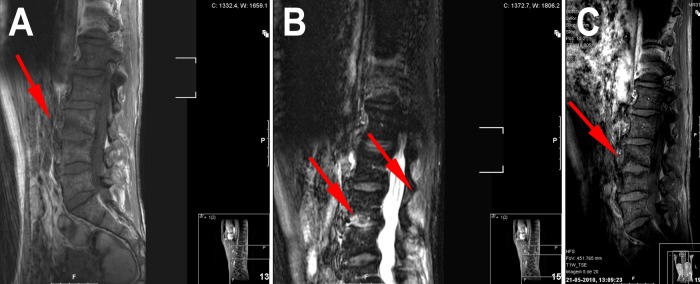
Sagital MRI: T1 (A) shows at L3–L4 level a hypointense signal in the vertebral marrow adjacent to the inferior L3 endplate, with loss of endplate definition on the superior side of the disc. Axial stir (B) shows hyperintense signal in the endplate anal disc (anterior two thirds). Postgadolinium axial T1 (C) shows enhancing signal in the endplate.
He recovered slowly, the pain decreased gradually and he restarted walking. The antibiotics were withdrawn after a total of 9 weeks.
Outcome and follow-up
On day 63 of admission, all antibiotics, prednisolone and arginine vasopressine had been suspended and neurological examination remained normal. Anticonvulsivants were withdrawn 6 months after discharge and, 3 years later he has completely recovered without any neurological deficit.
Discussion
This complex case highlights the vascular inflammation in the central nervous system (CNS) as a key issue in pneumococcal meningitis. Case death rates and risk of sequelae following meningitis are reported to be higher for S pneumoniae than Neisseria meningitidis or Haemophilus influenzae.5 6 7 12 Even if correct antimicrobial therapy is initially chosen, the proinflammatory cascade triggered by S pneumoniae and self-perpetuated by a dysregulated host inflammatory response will trigger mediators with vascular toxicity resulting in vasculitis syndromes.2 4 7 13 CNS vasculitis involvement has been reported to include seizures (27.6%), diffuse brain swelling (28.7%), hydrocephalus (16.1%), hearing loss (19.5%) and ischaemic or haemorrhagical brain damage (21.8%), being 67% of the ischaemic injuries caused by identified arteritis.2 7
The diagnosis of transient CDI in our patient was based on polyuria, polydipsia and inappropriately low plasma levels of arginine vasopressin in response to rise in plasma osmolality and a response to low-dose subcutaneous aqueous antidiuretic horomone (ADH) injection.9–11 Meningitis is often associated with the syndrome of inappropriate antidiuretic hormone secretion, contrasting with CDI which is an exceptional complication of meningitis and has been reported almost exclusively in paediatric patients.9–11 Greger et al14 found that 9% of children with bacterial meningitis develop CDI and that group B streptococci, H influenzae and S pneumonia were involved.9 We have only found one report in the literature of S pneumoniae meningitis-induced CDI in the adult.9 In addition, other occasionally reported aetiological agents of infection-precipitated CDI in adults include Mycobacterium tuberculosis, Blastomycosis, Cryptococcus neoformans, Escherichia coli, toxoplasmosis, herpes simplex and syphilis meningitis.9–11
The mechanism by which arginine vasopressin secretion is affected in bacterial meningitis is unknown. Two mechanisms have been suggested: infiltration of pituitary gland or pituitary stalk by pneumococcal infection and involvement of an autoinflammatory process that can cause enlargement of the pituitary gland due to lymphocytic inflammation.9 10 15 16 Although rare, the recognition of this disorder is essential because CDI can be life-threatening with the rapid development of severe hypernatraemia and, moreover, responds well to administration of synthetic ADH. We think that the main mechanism that led to our patient severe neurological manifestations was an inflammatory response and consequent vasculitis: he responded well to steroids and then he got worse after early steroid withdrawal on day 8.
Despite our increasing understanding of the pathophysiological cascades involved in bacterial meningitis, to date only dexamethasone has demonstrated its clinical efficacy as adjunctive therapy.2 7 17 Randomised controlled trials support corticosteroids therapy in children with H influenzae meningitis due to its proven improved long-term outcome.18 19 Although some authorities recommend dexamethasone use for patients with severely impaired mental status or high intracranial pressure, the benefits of such adjunctive therapy are far less clear in adult patients suffering from pneumococcal meningitis.2 In a recent quantitative review that included results of five clinical trials, treatment with corticosteroids was associated with a significant reduction in mortality and neurological sequelae.20–22 Several other studies have shown that steroid therapy is effective on preventing complications, but only when given very early in the course of bacterial meningitis, prior or at the time of the first dose of antibiotics.2 17 19 21 There is no scientific evidence supporting prolonged steroid treatment (>4 days) in pneumococcal meningitis, but some anecdotal reports hypothesised a rebound inflammatory effect after steroid withdrawal and that metalloproteinase 9 might have a rule on the vasculitic poststeroid inflammation.7 8 Those cases, and ours, remind us that some immunosuppression could be beneficial for specific delayed complications related to vascular inflammation and that a slower withdrawal should be considered.21
Although this was not the mainstay of this case, as infectious spondylodiscitis in adults are not rare events, vertebral osteomyelitis, due to this pathogen, has rarely been reported.23 24 In fact, the main causative organisms are staphylococci (40–60%), tuberculosis (20%) and Gram-negative bacteria. Although S pneumoniae is a virulent and invasive micro-organism and the third most common pathogen in the blood, pneumococcal osteomyelitis in adult is a rare event (<10%).23–25 Spondylodiscitis usually occurs as a result of haematogenous spread from an infective focus, not often identified, and its main location is lumbar (63%), followed by cervical (25%) and dorsal (22%).23 The course may be acute or chronic, but the lack of specific symptoms usually results in delayed diagnosis leading to potentially high morbidity and mortality.26 In this case, the degenerative changes of the lumbar spine may have contributed to the development of spondylodiscitis.23
At this point, we must acknowledge that although this complex and rare case demonstrates how IPD can be potentially life-threatening, the steroid therapy might have an important role, and fortunately performed well.
Learning points.
Invasive pneumococcal disease can be potentially life-threatening and needs prompt diagnosis and effective antibiotic treatment.
Cerebral vasculitis might be a late-onset complication of invasive pneumococcal disease.
Adjunctive treatment with steroids showed beneficial effect in suppressing the complications caused by inflammatory vasculitis, not only early on the course of the disease.
Although rare, central diabetes insipidus can occur as a complication of cerebral vasculitis in invasive pneumococcal disease.
Spondylodiscitis due to streptococcus pneumonia is extremely uncommon and can occur later in the course of the pneumococcal invasive disease
Footnotes
Contributors: SR and VD were involved in the conception and design, acquisition of data or analysis and interpretation of data and involved in drafting the article or revising it critically for important intellectual content. RMF and TM finalised the approval of the version published.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Pilishvili T, Noggle B, Moore M. Pneumococcal disease. In: Roush SW, McIntyre L, Baldy LM, eds. Centers for Disease Control and Prevention, Manual for the Surveillance of Vaccine-Preventable Diseases, 4th edn. Atlanta, GA: Centers for Disease Control and Prevention, 2008. [Google Scholar]
- 2.Auburtin M, Porcher R, Bruneel F, et al. Pneumococcal meningitis in the intensive care unit: prognostic factors of clinical outcome in a series of 80 cases. Am J Respir Crit Care Med 2002;2013:713–7 [DOI] [PubMed] [Google Scholar]
- 3.Harboe ZB, Thomsen RW, Riis A, et al. Pneumococcal serotypes and mortality following invasive pneumococcal disease: a population-based cohort study . PLoS Med 2009;2013:e1000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Public health notifiable disease management guidelines Invasive pneumococcal disease. Canada:Alberta Health and Wellness, 2011. http://www.health.alberta.ca/documents/Guidelines-Pneumococcal-Disease-Invasive-IPD-2011.pdf [Google Scholar]
- 5.Randle E, Ninis N, Inwald D. Invasive pneumococcal disease. Arch Dis Child Educ Pract Ed 2011;2013:183–90 [DOI] [PubMed] [Google Scholar]
- 6.Taylor SN, Sanders CV. Unusual manifestations of invasive pneumococcal infection. Am J Med 1999;2013:12S–27S [DOI] [PubMed] [Google Scholar]
- 7.Pugin D, Copin JC, Goodyear MC, et al. Persisting vasculitis after pneumococcal meningitis. Neurocrit Care 2006;2013:237–40 [DOI] [PubMed] [Google Scholar]
- 8.Lefebvre N, Carre AC, Delabranche X, et al. [Implication of dexamethasone adjunctive therapy after the onset of cerebral vasculitis in Streptococcus pneumoniae meningitis.] Med Mal Infect 2007;2013:118–20 [DOI] [PubMed] [Google Scholar]
- 9.Franco-Paredes C, Evans J, Jurado R. Diabetes insipidus due to Streptococcus pneumoniae meningitis. Arch Intern Med 2001;2013:1114–15 [DOI] [PubMed] [Google Scholar]
- 10.Juffermans NP, Verbon A, Van der Poll T. Diabetes insipidus as a complication of cryptococcal meningitis in an HIV-infected patient. Scand J Infect Dis 2002;2013:397–8 [DOI] [PubMed] [Google Scholar]
- 11.Kabakuş N, Yilmaz B, Aydinoğlu H, et al. Transient diabetes insipidus following Escherichia coli meningitis complicated by ventriculoperitoneal shunt. J Endocrinol Invest 1999;2013:800–2 [DOI] [PubMed] [Google Scholar]
- 12.Shackley F, Knox K, Morris JB, et al. Outcome of invasive pneumococcal disease: a UK based study. Oxford Pneumococcal Surveillance Group. Arch Dis Child 2000;2013:231–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mumtaz S, Fowler MR, Gonzales-Toledo E, et al. Central nervous system vasculitis. Curr Clin Neurol 2005;257–68 [Google Scholar]
- 14.Greger NG, Kirkland RT, Clayton GW, et al. Central diabetes insipidus. 22 years’ experience. Am J Dis Child 1986;2013:551–4 [DOI] [PubMed] [Google Scholar]
- 15.Lam KS, Sham MM, Tam SC, et al. Hypopituitarism after tuberculous meningites in childhood. Ann Intern Med 1993;2013:701–6 [DOI] [PubMed] [Google Scholar]
- 16.Imura H, Nakao K, Shimatsu A, et al. Lymphocytic infundibuloneurohypothysitis as a cause of central diabetes insipidus. N Engl J Med 1993;2013:683–9 [DOI] [PubMed] [Google Scholar]
- 17.de Gans J, van de Beek D. European Dexamethasone in Adulthood Bacterial Meningitis Study Investigators. Dexamethasone in adults with bacterial meningitis. N Engl J Med 2002;2013:1549–56 [DOI] [PubMed] [Google Scholar]
- 18.Lebel MH, Freij BJ, Syrogiannopoulos GA, et al. Dexamethasone therapy for bacterial meningitis. Results of two double-blind, placebo-controlled trials. N Engl J Med 1988;2013:964–71 [DOI] [PubMed] [Google Scholar]
- 19.Odio CM, Faingesicht I, Paris M, et al. The beneficial effects of early dexamethasone administration in infants and children with bacterial meningitis. N Engl J Med 1991;2013:1525–31 [DOI] [PubMed] [Google Scholar]
- 20.van de Beek D, de Gans J, McIntyre P, et al. Steroids in adults with bacterial meningitis: a systematic review. Lancet Infect Dis 2004;2013:139–43 [DOI] [PubMed] [Google Scholar]
- 21.van de Beek D, de Gans J, Tunkel A, et al. Community-acquired bacterial meningitis in adults. N Engl J Med 2006;2013:44–53 [DOI] [PubMed] [Google Scholar]
- 22.Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004;2013:1267–84 [DOI] [PubMed] [Google Scholar]
- 23.Gómez Rodríguez N, Durán Muñoz O. [Pneumococcal spondylodiscitis and pleural effusion. Report of a case and literature review]. An Med Interna 2007;2013:27–30 [DOI] [PubMed] [Google Scholar]
- 24.Grammatico L, Besnier JM. [Infectious spondylodiscitis]. Rev Prat 2007;2013:970–8 [PubMed] [Google Scholar]
- 25.Turner DP, Weston VC, Ispahani P. Streptococcus pneumoniae spinal infection in Nottingham, United Kingdom: not a rare event. Clin Infect Dis 1999;2013:873–81 [DOI] [PubMed] [Google Scholar]
- 26.Kapsalaki E, Gatselis N, Stefos A, et al. Spontaneous spondylodiscitis: presentation, risk factors, diagnosis, management, and outcome. Int J Infect Dis. 2009;2013:564–9 [DOI] [PubMed] [Google Scholar]


