Abstract
Polyamines are substances involved in many aspects of cell growth, division, and differentiation. Because of the metabolic differences between host cells and parasite cells, polyamine metabolism has been considered as a potential target for the chemotherapy of parasitic diseases. The aim of this work was to evaluate the schistosomicidal activity of different N-alkylated diamines (3a–3h), amino alcohols (4a–4d), and glycosylated amino alcohols (10a–10d). Compounds were prepared by synthetic methods and submitted to in vitro evaluation against adult worms of Schistosoma mansoni. At 100 μM, 3b, 3e, and 3h as well as 4a, 4b, 4d, 10a, 10b, and 10d resulted in 100% mortality of adult schistosomes. Compound 3d (12.5 to 100 μM) caused the death of 100% of both male and female adult schistosomes, while 3f (12.5 to 100 μM) resulted in 100% mortality of only male adult worms, whereas no mortality in female worms was observed. Compounds 3d and 3f were also able to reduce viability and decrease production of developed eggs in comparison with the negative control group. Diamines 3d and 3f may represent useful lead compounds for further optimization in order to develop new schistosomicidal agents.
1. Introduction
Human schistosomiasis is a chronic liver and intestinal parasitic disease caused by blood flukes of the genus Schistosoma, mainly S. mansoni [1]. Schistosomiasis, endemic in approximately 77 countries, is considered to be one of the most significant neglected tropical diseases in the world, affecting more than 200 million people worldwide [2]. The only drug used in treatment of schistosomiasis is praziquantel (PZQ), which does not prevent reinfection and is inactive against juvenile schistosomes [2, 3]. In addition, there is a considerable concern about the development of praziquantel resistance [3, 4]. This scenario emphasizes the increasing need for the development of novel and inexpensive drugs against schistosomiasis. In order to provide new hit/lead structures, which can be used in drug development to control schistosomiasis, the search for schistosomicidal compounds, mainly from natural sources, has been intensified in the last years [5–8].
Polyamines are substances that occur widely in biological material and are thought to be involved in many aspects of cell, such as growth, division, and differentiation [9]. Because of the metabolic differences between host cells and parasite cells, polyamine metabolism has been considered as a potential target for the chemotherapy of parasitic diseases [10].
Regarding the activity of amine compounds, several authors have shown the antiparasitic activity of different N-alkylated diamines and amino alcohols [10–17]. In a previous study, it was shown that lipophilic diamine and amino alcohol derivatives display inhibitory effects on promastigote forms of Leishmania chagasi and Leishmania amazonensis [12, 16]. It was also reported that this series of compounds showed trypanocidal activity against trypomastigotes forms of Trypanosoma cruzi, showing that compounds containing alkyl chains with 12 carbon atoms displayed similar activity to the reference drug crystal violet [17]. Moreover, Penido et al. reported the in vitro and in vivo schistosomicidal activity of a series of alkylaminothiosulfuric compounds [18].
Considering the design of novel antiparasitic drugs based on polyamine compounds and amino alcohol derivatives, lipophilicity is an important parameter. The introduction of long alkyl chains may enhance the ability of compounds in interacting with membrane lipids, allowing their penetration into the parasite and, consequently, modifying either the polyamine transport or the metabolism of the parasite. Also, the insertion of carbohydrates into amino alcohol molecules may be useful for disturbing cell integrity, since glycosylated derivatives could be considered nonionic surfactants compounds [9–17, 19].
In this context, in continuation of our search for bioactive schistosomicidal compounds [20, 21] and on the basis of their antiparasitic potential, this work describes the in vitro schistosomicidal activity of a series of lipophilic diamines and amino alcohols, as well as glycosylated amino alcohols derivatives against adult worms of Schistosoma mansoni.
2. Materials and Methods
2.1. General Methods
IR spectra were recorded using a BOMEM-FTIR MB102 spectrometer (AABB Bomem Inc., QC, Canada). 1H and 13C NMR spectra were recorded on Bruker Advance DRX300 spectrometer (Bruker Corporation, Billerica, MA, USA). Thin-layer chromatography (TLC) was performed on glass plates and silica gel sheets (Silica Gel F254; Merck, Whitehouse Station, NK, USA), visualized with iodine vapor, and/or revealed with ethanolic H2SO4 solution. Column chromatography was carried out on silica gel 60 (E. Merck 70–230 mesh). Solvents were purchased from Vetec Química (Vetec, Xerem, RJ, Brazil) and were distilled prior to use. Reagents were purchased from Aldrich (Sigma Aldrich, Saint Louis, MI, USA) and used without further purification. Log P was calculated using Chemdraw Ultra 12 software (trial version).
2.2. Synthesis of Alkylated Diamines and Amino Alcohols
N-mono, N,N′-dialkylated diamines (Scheme 1) and N-monoalkylated amino alcohols (Scheme 2) were prepared using a similar methodology previously described [10–14]. For the preparation of the N-mono and N,N′-dialkylated diamines (Scheme 1), alcohols 1a–e were first mesylated in pyridine, leading to compounds 2a–e. These mesylated derivatives were then treated with 1,2-ethanediamine or 1,3-propanediamine in ethanol under reflux, furnishing the desired compounds 3a–3h. For the preparation of N-monoalkylated amino alcohols 4a–d (Scheme 2), the mesylated derivative 2e was treated with commercial amino alcohols (diethanolamine, 2-amine-2-methylpropan-1-ol, ethanolamine, and 3-aminopropan-1-ol). For the obtainment of N-alkylated compounds 6a–c (Scheme 3) alkyl chlorides 5a–c were treated with ethanolamine in ethanol at reflux for 24 h furnishing the desired compounds. The alkylated diamines and amino alcohols were purified by column chromatography or recrystallization. The structures were assigned by 1H NMR and 13C NMR experiments [10–14].
Scheme 1.
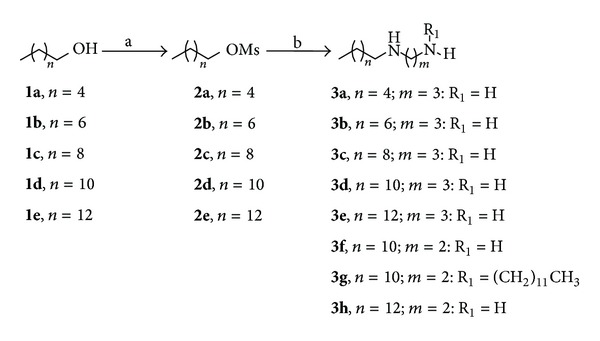
Synthesis of N-alkylated and N,N′-dialkylated diamines: (a) MsCl, CH2Cl2, Py, 0°C to room temperature; (b) diamine, EtOH reflux.
Scheme 2.
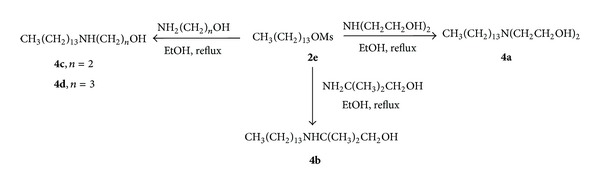
Synthesis of N-alkylated amino alcohols.
Scheme 3.
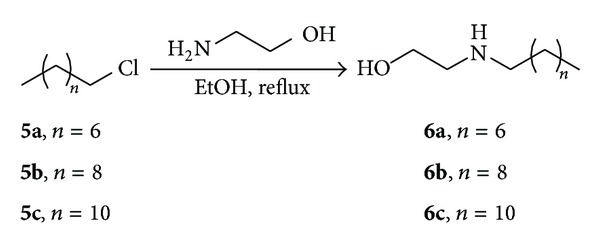
Synthesis of N-alkylated amino alcohols.
2.3. Synthesis of Glycosylated Derivatives
D-Galactose 7 was converted into 6-O-[2,3-epoxypropyl]-1,2 : 3,4-di-O-isopropylidene-α-D-galactopyranose 9 according to the literature procedure [22, 23] and then treated with amino alcohols 4c (Scheme 2) e 6a–c (Scheme 3) in presence of tetrabutyl ammonium bromide (TBAB) in EtOH at room temperature (Scheme 4). No attempt was made to determine the stereochemistry of compounds 10a–d as they were prepared from the corresponding racemic epichlorohydrin. All the compounds were purified by column chromatography. The structures were assigned by infrared and 1H and 13C NMR experiments [22, 23].
Scheme 4.
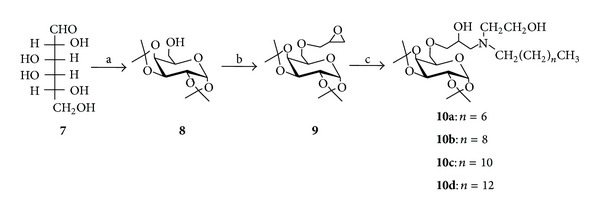
Preparation of D-galactose derivatives. Reagents and conditions: (a) acetone, H2SO4, ZnCl2 (58%); (b) NaOH 40%, TBAB, Epichlorohydrin, THF (94%); (c) CH3(CH2)nCH2NHCH2CH2OH, EtOH, TBAB, rt (51–61%).
2.4. Parasite Culture and Maintenance
The LE (Luiz Evangelista) strain of S. mansoni was maintained by passage through Biomphalaria glabrata snails and Balb/c mice. After eight weeks, S. mansoni adult worms (male and female) were recovered under aseptic conditions from mice previously infected with 200 cercariae by perfusion of the livers and mesenteric veins [20, 21]. The worms were washed in RPMI 1640 medium (Invitrogen), kept at pH 7.5 with HEPES 20 mM, and supplemented with penicillin (100 UI·mL−1), streptomycin (100 μg·mL−1), and 10% bovine fetal serum (Gibco). After washing, one pair of adult worms was transferred to each well of a 24-well culture plate containing 2 mL of the same medium and incubated at 37°C in a humid atmosphere containing 5% CO2 prior to use. All experiments were authorized by the Ethical Committee for Animal Care of University of São Paulo (Approval no.: 021/2009, June 2, 2009) in accordance with the national and international accepted principles for laboratory animal use and care.
2.5. In Vitro Studies with S. mansoni
For the in vitro test with S. mansoni, a preliminary screening of compounds was performed at 100 μM [20, 21]. The active samples were further studied at lower concentrations. Samples were dissolved in DMSO and used at concentrations ranging from 12.5 to 100 μM (compounds). Solutions of samples were added to the RPMI 1640 medium containing one adult worm pair after a period of 24 h of adaptation to the culture medium. The parasites were kept for 72 h and monitored every 24 h in order to evaluate their general condition (motor activity and mortality rate), egg production, and egg development. Significant alteration in motor activity was defined as minimal movement observed for 1 minute [5–8]. The worms were considered dead when no movement was observed for at least 2 minutes of examination [20, 21]. Changes in the pairing were also evaluated using an inverted microscope (Leitz) [5–8, 20, 21]. All experiments were carried out in quadruplicate and repeated at least four times, using 10 μM praziquantel (PZQ) as positive control group and RPMI 1640 medium and RPMI 1640 with 0.4% DMSO as negative control groups.
2.6. Viability Assay
Pairs of adult worms were incubated for 72 h with active compounds (12.5, 25, 50, and 100 μM), and their viability was assessed using the MTT assay [20, 21]. After incubation, each pair of adult worms was placed individually into wells (96-well plates) containing 100 μL of phosphate-buffered saline (PBS) with 5 mg of MTT per milliliter for 30 min at 37°C. The solution was carefully removed and replaced with 200 μL of DMSO, and the worms were allowed to stand in DMSO at room temperature for 1 h. The absorbance was read at 550 nm using as negative control groups RPMI 1640 medium and RPMI 1640 with 0.4% DMSO. PZQ (10 μM) was used as positive control group. The percentage of viability was calculated in relation to the negative control group.
2.7. Statistical Analysis
Results were expressed as mean ± S.E.M. Data were statistically analyzed by one-way analysis of variance, followed by Dunnett's test, with the level of significance set at P < 0.05.
3. Results and Discussion
Alkylated diamines and amino alcohols have been reported to possess a wide range of biological activities that include antibacterial, leishmanicidal, and trypanocidal [10–17]. However, to our knowledge, the schistosomicidal activity of alkylated diamines and amino alcohols is now being reported for the first time in this study. In this work, several diamines and amino alcohols compounds that had been reported to possess antiparasitic and/or antimicrobial activities were selected for our antischistosomal experiments.
The compounds 3a–3e, derived from 1,3-propanediamine, with lipophilic chain from 4 to 12 carbon atoms, were synthesized to evaluate the influence of lipophilicity on activity (Scheme 1). To evaluate the influence of the spacing between nitrogen atoms on schistosomicidal activity, the compounds 3f–h, derived from 1,2-ethanediamine, were synthesized (Scheme 1). The amino alcohols 4a–4d with lipophilic chains of 14 carbon atoms were synthesized to evaluate the influence of amino alcohol groups on schistosomicidal activity (Scheme 2). On the other hand, glycosylated amino alcohols (10a–10d) (Scheme 4) were synthesized to evaluate the influence of the carbohydrate subunit in the schistosomicidal activity [22, 23].
In a preliminary survival of 56-day-old adult worms of S. mansoni test, alkylated diamines (3a–3h), amino alcohols (4a–4d), and glycosylated amino alcohols (10a–10d) were tested at 100 μM. As can be observed in Table 1, diamines 3b, 3d, 3e, 3f, and 3 h as well as the amino alcohols 4a, 4b, 4d, 10a, 10b, and 10d showed significant schistosomicidal activities when tested at 100 μM, causing the death of 100% of S. mansoni adult worms. No significant results were found for alkylated diamines 3a, 3c, 3g, and amino alcohols 4c and 10c, which were not able to kill all adult parasites. As shown in Table 1, PZQ (10 μM) caused 100% mortality, whereas no effect was observed in worms in the control (RPMI 1640 medium) and vehicle (RPMI medium plus 0.4% DMSO) groups.
Table 1.
In vitro effects of alkylated diamines and amino alcohols against adult worms of S. mansoni.
| Samplesb | % worm separatione | % worm mortalitye | Significant reduction in motor activity (%)e | log(P) |
|---|---|---|---|---|
| Controla | 0.0 | 0.0 | 0.0 | |
| 0.4% DMSO | 0.0 | 0.0 | 0.0 | |
| PZQc | 0.0 | 100 | 0.0 | |
| Compoundsd | ||||
| 3a | 0.0 | 0.0 | 0.0 | 1.15 |
| 3b | 50 | 100 | 0.0 | 1.99 |
| 3c | 0.0 | 0.0 | 0.0 | 2.82 |
| 3d | 100 | 100 | 50 | 3.65 |
| 3e | 75 | 100 | 0.0 | 4.49 |
| 3f | 100 | 100 | 50 | 3.55 |
| 3g | 75 | 0.0 | 100 | 8.65 |
| 3h | 25 | 100 | 25 | 4.38 |
| 4a | 0.0 | 100 | 25 | 4.62 |
| 4b | 50 | 100 | 50 | 5.30 |
| 4c | 0.0 | 0.0 | 75 | 4.76 |
| 4d | 0.0 | 100 | 25 | 4.87 |
| 10a | 0.0 | 100 | 50 | 2.55 |
| 10b | 0.0 | 100 | 50 | 3.39 |
| 10c | 0.0 | 0.0 | 0.0 | 4.22 |
| 10d | 0.0 | 100 | 50 | 5.06 |
aRPMI 1640; bperiod of incubation: 72 h; ctested at concentration of 10 μM; dtested at concentration of 100 μM; epercentages relative to the 8 adult worm pairs investigated. Slight was defined as a reduction in movement compared with the negative control. Significant was defined as minimal movement observed for 1 minute. Dead worm was defined when no movement was observed for at least 2 minutes of examination. log(P) values were calculated using Chemdraw Ultra 12 software (trial version).
A correlation between biological activity and hydrophobic character is frequently observed. The partition coefficient P of a compound in an n-octanol/water system represents the hydrophobic properties of this compound and can be determined experimentally or calculated. Considering the number of dead worms for the compounds, at a concentration of 100 μM, a correlation between number of dead worms (%) and log (P) may be established (Table 1). All active compounds, at 100 μM, showed log (P) between 1.99 and 5.30, including compounds 3d (log P of 3.65) and 3f (log P of 3.65), excepting compounds 3c and 4c, which were inactive. Interestingly, the diamine 3c, with intermediate lipophilic chain, as well as compounds 3a (log P of 1.15) and 3g (log P of 8.65), which have lower and higher lipophilic chains, respectively, were inactive.
The active compounds in preliminary assays were further tested at lower concentrations (ranging from 12.5 to 50 μM), as presented in Table 2. Regarding mortality rate, when compound 3d was tested at 12.5 to 50 μM, it caused the death of 100% of both male and female S. mansoni adult worms, after 24 h of incubation. In contrast, the exposure to 12.5 to 50 μM of compound 3f resulted in 100% mortality of male adult worms, whereas no mortality in the female worms was observed. Similarly, glycosylated amino alcohols 10b and 10d, at 25 to 50 μM, caused the death of 100% of male adult worms and 25% mortality of female schistosomes, but at 12.5 μM, no impact on mortality of adult worms was observed after exposure to 10b and 10d. Additionally, 4a and 10a (50 μM) were able to cause 75% mortality of male and 25% mortality of female adult worms. Moreover, no significant effects on mortality were found after incubation with compounds 3b, 3e, 3h, 4b, and 4d at concentrations of 12.5 to 50 μM (Table 2), and morphological analysis revealed no tegumentary changes after incubation of both male and female S. mansoni adult worms with any tested compounds (data not shown).
Table 2.
In vitro effects of active diamines and amino alcohols against adult worms of S. mansoni.
| Samplesb | % worm separationd | % worm mortalityd | Significant reduction in motor activity (%)d | |
|---|---|---|---|---|
| M | F | |||
| Controla | 0.0 | 0.0 | 0.0 | 0.0 |
| 0.4% DMSO | 0.0 | 0.0 | 0.0 | 0.0 |
| PZQc | 0.0 | 100 | 100 | 100 |
| 3b | ||||
| 12.5 μM | 0.0 | 0.0 | 0.0 | 0.0 |
| 25 μM | 0.0 | 0.0 | 0.0 | 0.0 |
| 50 μM | 0.0 | 0.0 | 0.0 | 0.0 |
| 3d | ||||
| 12.5 μM | 50 | 100 | 100 | 0.0 |
| 25 μM | 50 | 100 | 100 | 0.0 |
| 50 μM | 50 | 100 | 100 | 0.0 |
| 3e | ||||
| 12.5 μM | 50 | 0.0 | 0.0 | 0.0 |
| 25 μM | 100 | 0.0 | 0.0 | 0.0 |
| 50 μM | 100 | 0.0 | 0.0 | 0.0 |
| 3f | ||||
| 12.5 μM | 50 | 100 | 0.0 | 0.0 |
| 25 μM | 50 | 100 | 0.0 | 0.0 |
| 50 μM | 50 | 100 | 0.0 | 0.0 |
| 3h | ||||
| 12.5 μM | 0.0 | 0.0 | 0.0 | 0.0 |
| 25 μM | 0.0 | 0.0 | 0.0 | 0.0 |
| 50 μM | 0.0 | 0.0 | 0.0 | 0.0 |
| 4a | ||||
| 12.5 μM | 0.0 | 0.0 | 0.0 | 0.0 |
| 25 μM | 0.0 | 0.0 | 0.0 | 0.0 |
| 50 μM | 0.0 | 75 | 25 | 0.0 |
| 4b | ||||
| 12.5 μM | 50 | 0.0 | 0.0 | 0.0 |
| 25 μM | 100 | 0.0 | 0.0 | 0.0 |
| 50 μM | 100 | 0.0 | 0.0 | 0.0 |
| 4d | ||||
| 12.5 μM | 0.0 | 0.0 | 0.0 | 0.0 |
| 25 μM | 0.0 | 0.0 | 0.0 | 0.0 |
| 50 μM | 0.0 | 0.0 | 0.0 | 0.0 |
| 10a | ||||
| 12.5 μM | 0.0 | 0.0 | 0.0 | 0.0 |
| 25 μM | 0.0 | 0.0 | 0.0 | 0.0 |
| 50 μM | 0.0 | 75 | 25 | 0.0 |
| 10b | ||||
| 12.5 μM | 0.0 | 0.0 | 0.0 | 0.0 |
| 25 μM | 0.0 | 100 | 0.0 | 0.0 |
| 50 μM | 0.0 | 100 | 25 | 0.0 |
| 10d | ||||
| 12.5 μM | 0.0 | 0.0 | 0.0 | 0.0 |
| 25 μM | 0.0 | 100 | 25 | 0.0 |
| 50 μM | 0.0 | 100 | 50 | 0.0 |
aRPMI 1640; bperiod of incubation: 72 h; ctested at 10 μM; dpercentages relative to the 8 adult worm pairs investigated. M: males; F: females. Slight was defined as a reduction in movement compared with the negative control. Significant was defined as minimal movement observed for 1 minute. Dead worm was defined when no movement was observed for at least 2 minutes of examination.
It is important to emphasize that male worms were more susceptible than female worms to the active compounds 10b, 10c, and especially for 3f. Interestingly, compound 3d, which is chemically similar to 3f, caused the death of 100% of adult schistosome with no distinction between male and female. A similar variation in drug susceptibility between male and female schistosomes has been observed with several antischistosomal drugs [4–8, 20, 21, 24]. Similarly, recent studies on the effects of the essential oil of Ageratum conyzoides, ginger extract (Zinger officinale), and PZQ on S. mansoni showed that male worms tended to be more susceptible than female worms [8, 24–27]. In contrast, results with artesunate showed higher survival rates for male than for female worms [28]. Remarkably, our data demonstrated that compound 3f showed an optimal in vitro activity against adult stage of S. mansoni, exhibiting high differential sensitivity to male worms at all tested concentrations (12.5 to 50 μM).
On the other hand, no impact on worm motor activity was observed in the groups treated with no lethal concentrations of all active compounds (Table 2). Also, schistosomes maintained in RPMI 1640 medium or in RPMI medium plus 0.4% DMSO kept a normal motor activity. In contrast, all parasites belonging to the positive control group (PZQ, 10 μM) had a significant decrease in motor activity. In this regard, it has been reported that the motility of S. mansoni is associated with important neurotransmitters or neuromodulators such as serotonin, dopamine, acetylcholine, epinephrine, neuropeptides, and glutamate [29–31]. Thus, it is suggested that mortality rate of adult schistosomes exposed to these tested diamines and amino alcohols might not be associated with alterations in the neurotransmitter or neuromodulator system of the parasite.
In addition, considering human schistosomiasis, its pathology is not due directly to the adult worm but rather to the large numbers of eggs that become trapped in the host's tissues, during egg migration [25–29, 32]. Regarding this, the analysis of the effects of schistosomicidal compounds on the reproductive fitness of S. mansoni may be an important strategy used to discover new drugs [32, 33]. In this sense, the reproductive fitness of S. mansoni is assessed by (i) pairing, an indicator of the mating process; and (ii) egg production, an indicator of egg output per worm [32, 33]. According to the literature, the permanent pairing of the schistosomes couples in the blood system of their hosts vertebrates throughout their lifespan causes high rate of oviposition, which is responsible for the resulting immunopathological lesions, characterized by inflammation and fibrosis in the target organ [8, 34]. Furthermore, in order to evaluate the schistosomicidal effect of a drug, it is important to analyze several parameters, such as motility, oviposition, and mortality [25, 32]. Also, according to Moraes et al. (2011), compounds with schistosomicidal activity can be effective in different ways: prophylactically (causing the death of schistosomula), suppressively (inhibiting oviposition), and curatively (causing the death of the adult worm) [31–33].
Regarding changes in the pairing, all pairs of coupled adult worms were separated into individual male and female after incubation with 25 to 50 μM of compounds 3e and 4b, while 50% of the pairs remained coupled after exposure of compounds 3d (12.5 to 50 μM), 3e (12.5 μM), 3f (12.5 to 50 μM), and 4b (12.5 μM). PZQ (10 μM) caused 100% of death of the parasites without separation of worms.
After preliminary screenings and mortality analysis, the viability and egg development assays of adult S. mansoni worms were evaluated by incubation with different concentrations of the most active compounds 3d and 3f (12.5 to 100 μM) (Figure 1). In the groups treated with 3f (25 to 100 μM), the viability of adult worms was statistically similar to PZQ (10 μM) after 72 hours of incubation. Similarly, groups of adult worms treated with 3d (100 to 100 μM) displayed reduced viability compared to the positive control PZQ. It is important to point out that compounds 3d and 3f showed high activity in this assay, but a dose-response effect was not observed in comparison with the negative control group, treated with RPMI 1640 medium, in which the worms remained viable during 72 h of incubation (Figure 1).
Figure 1.
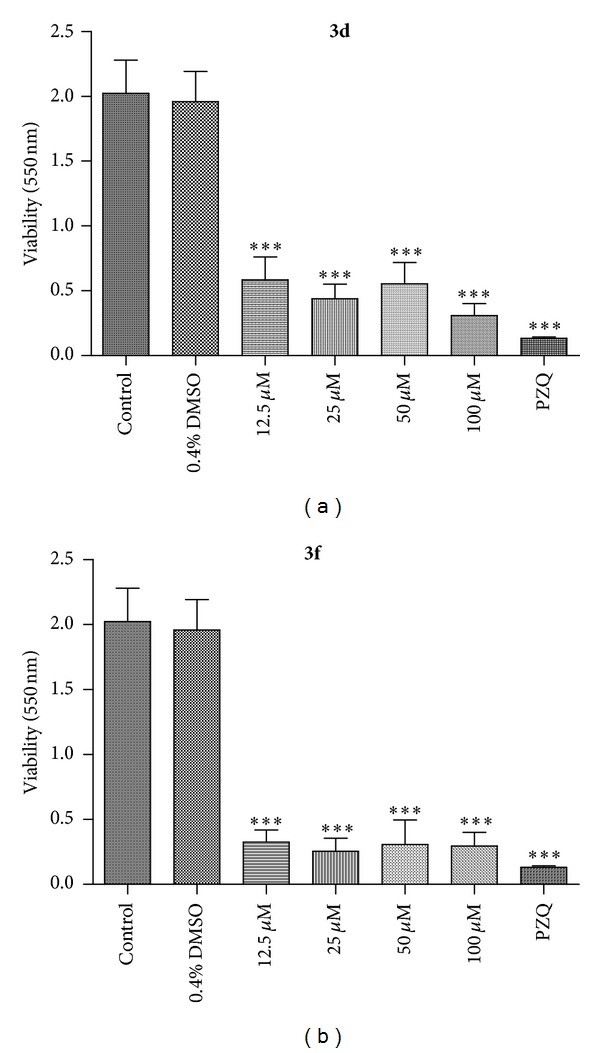
In vitro effects of active diamines 3d and 3f on the viability of the S. mansoni adult worms. Pairs of adult worms were treated with samples in different concentrations for 72 h, and the viability was measured by using the MTT assay at 550 nm. RPMI 1640 medium and 0.4% DMSO in RPMI 1640 medium were used as negative controls. Praziquantel (PZQ, 10 μM) was used as positive control group. The viability was expressed as mean of the absorbance values from four experiments. ***P < 0.001.
In order to evaluate the percentage of developed eggs produced by adult worms of S. mansoni, groups of parasites were incubated with 3d and 3f (12.5 to 100 μM) and monitored for 72 hours. As shown in Figure 2, compounds 3d (12.5 to 100 μM) and 3f (12.5 to 100 μM) showed significant decrease in the production of developed eggs when compared with the negative control group but not in a dose-response dependent manner. Despite the difference in groups, this in vitro bioassay showed a significant reduction in the mean egg development output after exposure to concentrations of the active compounds 3d and 3f. According to the literature, 20–30% of the eggs produced by adult worms in the first two days of in vitro culture went through the six development stages in five days until the formation of the miracidium inside could be considered as fully developed [5, 8, 24]. Also, the presence of S. mansoni eggs in the host tissues has been reported to be closely related to the pathology of human schistosomiasis, and egg production is responsible for the transmission of the parasite schistosome and the maintenance of its life cycle [8, 27]. In the present study, we observed that compounds 3d and 3f reduced egg development output by affecting pairing or mortality of female worms. However, it is still unknown whether these antifecundity effects of compounds 3d and 3f were the result of more specific inhibition of reproductive processes.
Figure 2.
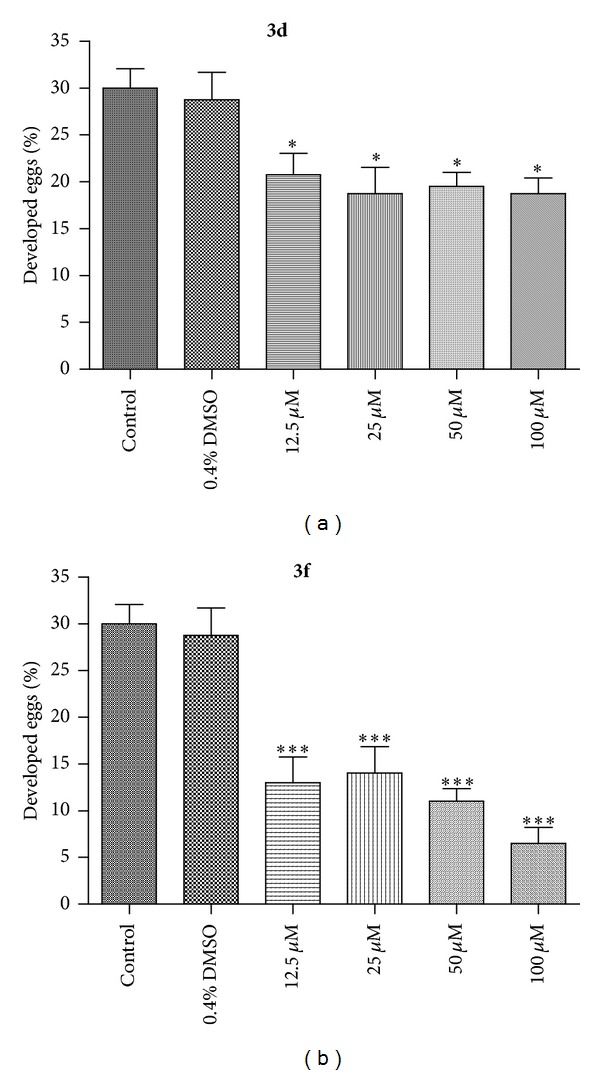
In vitro active diamines 3d and 3f on egg development (quantitative analysis of the development phenotype). After treatment, the eggs were microscopically examined and scored as developed or undeveloped based on the presence or absence of the miracidium. Data are presented as the mean of developed eggs from four separate experiments. *P < 0.05, ***P < 0.001.
Considering the most active compounds, the mechanism by which lipophilic diamines 3d and 3f exert their in vitro schistosomicidal effect is not clear. One of the possible targets for the diamines 3d and 3f actions is the damage of schistosome metabolism. It has been reported that polyamines are essential for cell proliferation and differentiation of some parasites. It has been shown that interfering with their function or biosynthesis, polyamines can block cellular growth [10, 11]. Also, it was suggested that diamines containing long alkyl chains, such as compound 3f, seem to impair the parasite biosynthetic metabolism, leading to parasite death [10–17]. However, taken all results together, even 3d and 3f have similar chemical structures; they may have different mechanisms involved in the parasite's death, since they affect in a different way male and female adult schistosomes. Due to the complexity of drug mechanisms and their mode of action, future biological experiments are necessary to clarify their mechanisms of action.
4. Conclusion
Diamines and amino alcohols were prepared by synthetic methods and submitted to in vitro evaluation against adult worms of Schistosoma mansoni. The present results indicate that alkylated diamines and amino alcohols are potentially antiparasitic compounds that may be useful starting points to find an ideal lead for the development of new schistosomicidal agents. We have identified some promising compounds that demonstrate high in vitro activity against adult schistosome, especially to male worms. However, further in vitro and in vivo studies are necessary to fully determine the potential chemotherapeutic efficacy of these compounds in the schistosome treatment.
Conflict of Interests
The authors declare that there is no conflict of interests.
Acknowledgments
The authors are grateful to FAPEMIG and FAPESP for financial support and to CAPES and CNPq for fellowships.
References
- 1.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Tropica. 2000;77(1):41–51. doi: 10.1016/s0001-706x(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Weekly Epidemiological Record. Vol. 86. Geneva, Switzerland: World Health Organization; 2011. Schistosomiasis: number of people treated worldwide in 2009; pp. 73–80. [Google Scholar]
- 3.Doenhoff MJ, Hagan P, Cioli D, et al. Praziquantel: its use in control of schistosomiasis in sub-Saharan Africa and current research needs. Parasitology. 2009;136(13):1825–1835. doi: 10.1017/S0031182009000493. [DOI] [PubMed] [Google Scholar]
- 4.Fallon PG, Tao L-F, Ismail MM, Bennett JL. Schistosome resistance to praziquantel: fact or artifact? Parasitology Today. 1996;12(8):316–320. doi: 10.1016/0169-4758(96)10029-6. [DOI] [PubMed] [Google Scholar]
- 5.Magalhães LG, Kapadia GJ, da Silva Tonuci LR, et al. In vitro schistosomicidal effects of some phloroglucinol derivatives from Dryopteris species against Schistosoma mansoni adult worms. Parasitology Research. 2010;106(2):395–401. doi: 10.1007/s00436-009-1674-8. [DOI] [PubMed] [Google Scholar]
- 6.Parreira NA, Magalhães LG, Morais DR, et al. Antiprotozoal, schistosomicidal, and antimicrobial activities of the essential oil from the leaves of Baccharis dracunculifolia . Chemistry and Biodiversity. 2010;7(4):993–1001. doi: 10.1002/cbdv.200900292. [DOI] [PubMed] [Google Scholar]
- 7.Magalhães LG, Machado CB, Morais ER, et al. In vitro schistosomicidal activity of curcumin against Schistosoma mansoni adult worms. Parasitology Research. 2009;104(5):1197–1201. doi: 10.1007/s00436-008-1311-y. [DOI] [PubMed] [Google Scholar]
- 8.Tonuci LRS, de Melo NI, Dias HJ, et al. In vitro schistosomicidal effects of the essential oil of Tagetes erecta . Brazilian Journal of Pharmacognosy. 2011;22(1):88–93. [Google Scholar]
- 9.Mastri C, Thorborn DE, Davies AJ, Ariyanayagam MR, Hunter KJ. Polyamine and thiol metabolism in Trypanosoma granulosum: similarities with Trypanosoma cruzi . Biochemical and Biophysical Research Communications. 2001;282(5):1177–1182. doi: 10.1006/bbrc.2001.4704. [DOI] [PubMed] [Google Scholar]
- 10.del Olmo E, Alves M, López JL, et al. Leishmanicidal activity of some aliphatic diamines and amino-alcohols. Bioorganic and Medicinal Chemistry Letters. 2002;12(4):659–662. doi: 10.1016/s0960-894x(01)00837-x. [DOI] [PubMed] [Google Scholar]
- 11.Labadie GR, Choi S-R, Avery MA. Diamine derivatives with antiparasitic activities. Bioorganic and Medicinal Chemistry Letters. 2004;14(3):615–619. doi: 10.1016/j.bmcl.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 12.da Costa CF, Coimbra ES, Braga FG, dos Reis RCN, da Silva AD, de Almeida MV. Preparation and antileishmanial activity of lipophilic N-alkyl diamines. Biomedicine and Pharmacotherapy. 2009;63(1):40–42. doi: 10.1016/j.biopha.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 13.de Souza NB, Rezende CAM, Toledo MS, et al. Antimalarial activity of an N-alkyl diamine. Letters in Drug Design and Discovery. 2011;8(4):371–374. [Google Scholar]
- 14.Giordani RB, Araújo DP, Duarte M, Zuanazzi JA, Tasca T, De Almeida MV. Anti-protozoal activity of diamine derivatives. Biomedicine and Pharmacotherapy. 2011;65(1):60–62. doi: 10.1016/j.biopha.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Giordani RB, de Almeida MV, Fernandes E, et al. Anti-Trichomonas vaginalis activity of synthetic lipophilic diamine and amino alcohol derivatives. Biomedicine and Pharmacotherapy. 2009;63(8):613–617. doi: 10.1016/j.biopha.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Coimbra ES, de Almeida MV, Junior COR, et al. Synthesis and antileishmanial activity of lipidic amino alcohols. Chemical Biology & Drug Design. 2010;75(2):233–235. doi: 10.1111/j.1747-0285.2009.00923.x. [DOI] [PubMed] [Google Scholar]
- 17.Júnior COR, Alves RO, Rezende CAM, et al. Trypanocidal activity of lipophilic diamines and amino alcohols. Biomedicine and Pharmacotherapy. 2010;64(9):624–626. doi: 10.1016/j.biopha.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Penido ML, Nelson DL, Vieira LQ, Coelho PM. Schistosomicidal activity of alkylaminooctanethiosulfuric acids. Memórias do Instituto Oswaldo Cruz. 1994;89(4):595–602. doi: 10.1590/s0074-02761994000400017. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann HO, Gercken G. Metabolism of 1-O-[1′-14C]octadecyl-sn-glycerol in Leishmania donovani promastigotes. Ether lipid synthesis and degradation of the ether bond. Molecular and Biochemical Parasitology. 1982;5(2):65–76. doi: 10.1016/0166-6851(82)90042-1. [DOI] [PubMed] [Google Scholar]
- 20.Pereira AC, Magalhães LG, Gonalves UO, et al. Schistosomicidal and trypanocidal structure-activity relationships for (±)-licarin A and its (−)- and (+)-enantiomers. Phytochemistry. 2011;72(11-12):1424–1430. doi: 10.1016/j.phytochem.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Ramalhete C, Magalhães LG, Rodrigues V, Mulhovo S, da Silva Filho AA, Ferreira MJ. In vitro schistosomicidal activity of balsaminol F and karavilagenin C. Planta Medica. 2012;78(18):1912–1917. doi: 10.1055/s-0032-1327832. [DOI] [PubMed] [Google Scholar]
- 22.Taveira AF, Hyaric ML, Reis EFC, et al. Preparation and antitubercular activities of alkylated amino alcohols and their glycosylated derivatives. Bioorganic and Medicinal Chemistry. 2007;15(24):7789–7794. doi: 10.1016/j.bmc.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 23.Tewari N, Tiwari VK, Tripathi RP, et al. Synthesis of galactopyranosyl amino alcohols as a new class of antitubercular and antifungal agents. Bioorganic and Medicinal Chemistry Letters. 2004;14(2):329–332. doi: 10.1016/j.bmcl.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Mitsui Y, Aoki Y. In vitro effect of current antimalarial drugs on the survival of paired Schistosoma mansoni adult worms and their egg production. Tropical Medicine and Health. 2010;38(2):69–73. [Google Scholar]
- 25.Moraes J, Almeida AAC, Brito MRM, et al. Anthelmintic activity of the natural compound (+)-limonene epoxide against Schistosoma mansoni . Planta Medica. 2013;79(3-4):253–258. doi: 10.1055/s-0032-1328173. [DOI] [PubMed] [Google Scholar]
- 26.Pica-Mattoccia L, Cioli D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. International Journal for Parasitology. 2004;34(4):527–533. doi: 10.1016/j.ijpara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Sanderson L, Bartlett A, Whitfield PJ. In vitro and in vivo studies on the bioactivity of a ginger (Zingiber officinale) extract towards adult schistosomes and their egg production. Journal of Helminthology. 2002;76(3):241–247. doi: 10.1079/JOH2002116. [DOI] [PubMed] [Google Scholar]
- 28.Mitsui Y, Miura M, Aoki Y. In vitro effects of artesunate on the survival of worm pairs and egg production of Schistosoma mansoni . Journal of Helminthology. 2009;83(1):7–11. doi: 10.1017/S0022149X08070235. [DOI] [PubMed] [Google Scholar]
- 29.Oliveira RN, Rehder VLG, Santos Oliveira AS, et al. Schistosoma mansoni: in vitro schistosomicidal activity of essential oil of Baccharis trimera (less) DC. Experimental Parasitology. 2012;132(2):135–143. doi: 10.1016/j.exppara.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 30.Taman A, Ribeiro P. Glutamate-mediated signaling in Schistosoma mansoni: a novel glutamate receptor is expressed in neurons and the female reproductive tract. Molecular and Biochemical Parasitology. 2011;176(1):42–50. doi: 10.1016/j.molbiopara.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Marks NJ, Maule AG. Neuropeptides in helminths: occurrence and distribution. Advances in Experimental Medicine and Biology. 2010;692:49–77. doi: 10.1007/978-1-4419-6902-6_4. [DOI] [PubMed] [Google Scholar]
- 32.Veras LM, Guimarães MA, Campelo YD, et al. Activity of epiisopiloturine against Schistosoma mansoni . Current Medicinal Chemistry. 2012;19(13):2051–2058. doi: 10.2174/092986712800167347. [DOI] [PubMed] [Google Scholar]
- 33.Moraes JD, Nascimento C, Lopes POMV, et al. Schistosoma mansoni: in vitro schistosomicidal activity of piplartine. Experimental Parasitology. 2011;127(2):357–364. doi: 10.1016/j.exppara.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 34.Knobloch J, Kunz W, Grevelding CG. Herbimycin A suppresses mitotic activity and egg production of female Schistosoma mansoni . International Journal for Parasitology. 2006;36(12):1261–1272. doi: 10.1016/j.ijpara.2006.06.004. [DOI] [PubMed] [Google Scholar]


