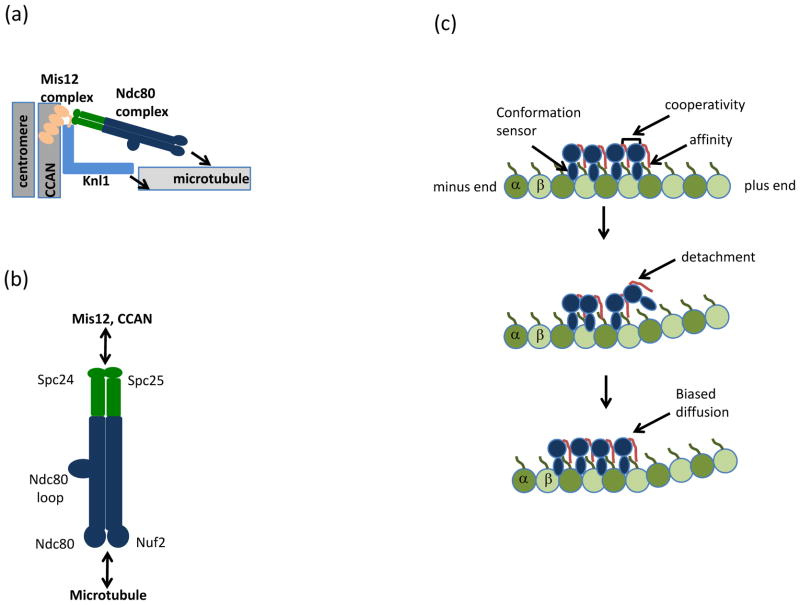
Figure 2. Architectural organization of the KMN network.
(a) Schematic of the Knl1-Mis12 complex-Ndc80 complex (KMN) network at the kinetochore. The four-subunit Mis-segregation 12 (Mis12) complex bridges Kinetochore null 1 (Knl1) and the Nuclear division cycle 80 (Ndc80) complex to the Conserved Centromere-Associated Network (CCAN) and underlying centromeric DNA. Arrows indicate microtubule binding activities in the Ndc80 complex and Knl1. (b) The Ndc80 complex, a dumbbell shaped heterotetramer, consists of a pair of microtubule-binding calponin-homology domains within the Ndc80 protein and Nuclear filamentous 2 (Nuf2) subunits (blue). The kinetochore domain is distal to the microtubule-binding domain and resides in the Spindle pole component 24 (Spc24) and Spc25 heterodimer (green). The subcomplexes interact via coiled-coil domains, which in the Ndc80 protein is interrupted by a loop that mediates protein-protein interactions. (c) Model for Ndc80 protein (blue) association with tubulin (green). Modified with permission from24. The Ndc80 protein has the unusual property of recognizing the region between α and β tubulin subunits, in contrast to most microtubule-binding proteins which bind microtubules once per tubulin dimer. Recognition of both intra- and inter-tubulin interfaces is thought to allow the Ndc80 complex to preferentially bind straight (top) versus the curled (middle) tubulin interfaces at disassembling microtubule tips. During detachment of the Ndc80 protein (middle) from curved microtubule structures, cooperativity between Ndc80 complexes (mediated by the Ndc80 tail, red) allows persistent microtubule and diffusion towards the microtubule minus-end (bottom). The positively charged Ndc80 tail also increases the affinity of the Ndc80 complex for microtubules via interaction with the negatively charged tubulin E-hook (green line) present in α and β tubulin subunits.