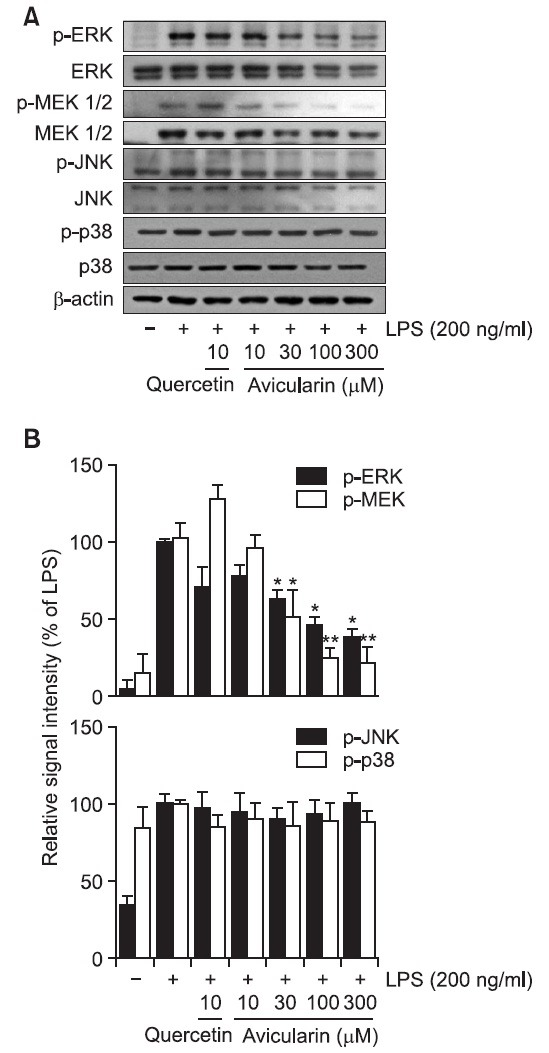
Fig. 6. Avicularin inhibited LPS-stimulated ERK signaling pathway in RAW 264.7 macrophage cells. RAW 264.7 cells were stimulated with 200 ng/ml LPS in the absence or presence of avicularin. (A) Western blot analysis was then performed to evaluate the activation of MAP kinases signaling pathways. (B) Ratio of phosphorylated forms to native forms was carried out by densitometric analysis. LPS-induced phosphorylation of ERK was significantly attenuated with aviculairn treatment, but phosphorylation of JNK and p38 was not decreased with avicularin treatment. Phosphorylation of MEK, upstream kinase of ERK, was accordingly attenuated with avicularin, suggesting that MEK/ERK might play an important role in the LPS-induced overactivation of RAW 264.7 cells. β-Actin was used as an internal control. Images are representative of three independent experiments that shows reproducible results. *p<0.05 and **p<0.01 indicate statistically significant differences from treatment with LPS alone.