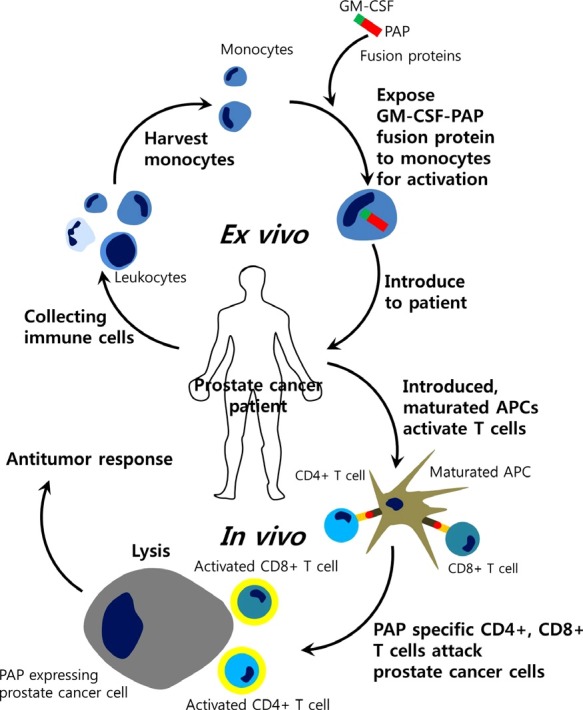
Fig. 4. Schematic diagram of Provenge trial. The stages of Sipuleucel-T treatment for patients with prostate cancer are shown. Sipuleucel-T treatment is similar to a dendritic cell (DC) vaccine. It is a United States Food and Drug Administration (FDA)-approved autologous cell-based immunotherapy that targets prostatic acid phosphatase (PAP) as a treatment for advanced prostate cancer. Modified from Garcia (2011) and Gerritsen (2012). GM-CSF: granulocyte-macrophage colony-stimulating factor; APC: antigen-presenting cells.