Abstract
Malaria, a mosquito-borne disease caused by Plasmodium species, causes substantial morbidity and mortality throughout the world. Plasmodium sporozoites mature in oocysts formed in the mosquito gut wall and then invade the salivary glands, where they remain until transmitted to the vertebrate host during a mosquito bite. The Plasmodium circumsporozoite protein (CSP) binds to salivary glands and plays a role in the invasion of this organ by sporozoites. We identified an Anopheles salivary gland protein, named CSP-binding protein (CSPBP), that interacts with CSP. Downregulation of CSPBP in mosquito salivary glands inhibited invasion by Plasmodium organisms. In vivo bioassays showed that mosquitoes that were fed blood with CSPBP antibody displayed a 25% and 90% reduction in the parasite load in infected salivary glands 14 and 18 days after the blood meal, respectively. These results suggest that CSPBP is important for the infection of the mosquito salivary gland by Plasmodium organisms and that blocking CSPBP can interfere with the Plasmodium life cycle.
Keywords: Anopheles gambiae, circumsporozoite protein, invasion, Sporozoite
Malaria, caused by various Plasmodium species, results in illness in >200 million individuals and in approximately 665 000 deaths annually throughout the world [1]. Mosquitoes of the genus Anopheles transmit Plasmodium organisms to humans. Female mosquitoes acquire gametocyte-stage parasites by feeding on an infected host. The parasites carry out fertilization in the gut, transform into ookinetes, and then form oocysts, which produce sporozoites. Oocyst-derived sporozoites must invade the mosquito salivary glands to be transmitted to a human host. Sporozoite invasion of the glands is mediated by specific receptor-ligand interactions [2–5]. This process probably occurs in 2 stages: first, sporozoites bind and penetrate the salivary gland basal lamina, and second, sporozoites interact with the membrane of the epithelial cells, leading to parasite internalization [6].
The circumsporozoite protein (CSP) is a glycosylphosphatidylinositol-anchored protein essential both for sporozoite development in the oocyst and invasion of the salivary glands [7, 8]. Additional parasite ligands necessary for sporozoite invasion of the salivary glands have been identified. The thrombospondin-related anonymous protein (TRAP) is important for attachment to and invasion of salivary glands by parasites [9]. Other proteins that have a role in salivary gland invasion include cysteine repeat modular proteins and apical membrane antigen/erythrocyte binding-like (MAEBL) [10, 11]. Attempts to identify candidate receptors for sporozoite invasion have been partially successful. A previous study reported the isolation of one such target antigen, Aedes aegypti salivary gland surface protein 1 (aaSGS1), and its apparent involvement in Plasmodium gallinaceum invasion and identified previously unknown Anopheles gambiae homologues of aaSGS1 [12]. In a separate set of experiments, monoclonal antibodies raised against A. gambiae salivary glands led to the identification of a 50-kDa protein, termed saglin [13, 14]. Saglin facilitates sporozoite invasion by interacting with TRAP [9]. While saglin may participate in the invasion of salivary glands by Plasmodium organisms, growing evidence suggests that several salivary gland molecules are likely to be involved in sporozoite entry into this organ. Further characterization of molecular interactions between sporozoite and salivary glands may clarify the invasion process and lead to novel targets to interrupt the malaria transmission cycle.
Plasmodium berghei is one of the many species of malaria parasites that infect mammals other than humans and provides an excellent laboratory model for studying Plasmodium organisms in mosquitoes and mice. We characterize a CSP-binding protein (CSPBP) from A. gambiae, invoke a pivotal role for CSPBP during sporozoite infection of salivary glands, and demonstrate that blocking CSPBP can interfere with the Plasmodium life cycle in mosquitoes.
METHODS
Mosquito Rearing and Infection
Mosquito (A. gambiae 4ARR strain) rearing and infection with the rodent parasite P. berghei were conducted as described elsewhere [15]. P. berghei strain ANKA, in which the HSP70 promoter controls the constitutive expression of green fluorescent protein (GFP), was used [16]. The parasite was maintained by mosquito transmission in A. gambiae, interspersed by a maximum of 2 serial passages in Swiss Webster mice. Mosquitoes aged 3–5 days fed on anesthetized P. berghei–infected Swiss Webster mice with abundant gametocyte-stage parasites.
Ethics Statement
Animals were housed and handled under standards set out in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal experimental protocol was approved by the Yale University Institutional Animal Care and Use Committee (protocol permit number 2008-07941). All animal experiments were performed in a biosafety level 2 facility, according to the regulations of Yale University.
RNA Interference (RNAi) Gene-Silencing Assays
CSPBP double-stranded RNAs (dsRNAs) were synthesized from polymerase chain reaction (PCR)–amplified fragments, using the T7 Megascript RNAi kit (Ambion) according to the manufacturer's protocol. Primer sequences are listed in Supplementary Table 1. GFP dsRNA was used as a control for nonspecific effects of dsRNA injection. A total of 1000 ng of dsRNA was injected into the thorax of cold-anesthetized 2-week-old female mosquitoes 11 days after their blood meal, using a nanoinjector (Nanoject II; Drummond) with a glass capillary needle, according to established methods [17]. Salivary gland sporozoites were harvested on day 18 after the blood meal. The mosquitoes were rinsed in 70% ethanol and washed in Dulbecco's modified Eagle's medium before salivary gland dissection.
Quantitative Reverse Transcription–PCR (qRT-PCR)
Mosquito salivary glands were dissected, and total RNA was isolated using an RNeasy kit (Qiagen). Genomic DNA was removed by on-column DNase digestion. RNA was converted into first-strand complementary DNA (cDNA), using the iScript RT-PCR kit (Bio-Rad). A total of 2 μL of cDNA synthesis reaction was analyzed by qPCR, using the SYBR green detection system (Applied Biosystems) with the Bio-Rad CFX96TM real-time system. The program consisted of an initial denaturing step at 95°C for 3 minutes and 40 amplification cycles at 95°C for 30 seconds, followed by 60°C for 1 minute. The A. gambiae ribosomal protein S7 (RPS7) was used as an internal control to normalize the amounts of RNA between the various samples. The gene-specific primers used for qRT-PCR are listed in Supplementary Table 1.
Quantification of Sporozoites in Mosquito Salivary Glands
The salivary gland sporozoite load was determined both microscopically and by qRT-PCR. To determine the proportion of internalized sporozoites among salivary gland–associated sporozoites, salivary glands were dissected out on day 18 after the blood meal and incubated with trypsin (50 μg/mL) in Medium 199 for 15 minutes at 37°C. Samples were then centrifuged for 5 minutes. The supernatant containing the attached sporozoites was collected, and the salivary glands were then ground to free internalized sporozoites. The glands were gently homogenized and spun at 80 ×g for 3 minutes to remove mosquito debris. The sporozoite-containing supernatant was removed, and sporozoites were counted by fluorescence microscopy in a hemocytometer.
Antibody Production
The N-terminal fragment of CSPBP was amplified from the mosquito salivary gland cDNA, using gene-specific primers (Supplementary Table 1). The PCR product was subcloned into the pGEX-6P2 vector (Invitrogen, CA) and transfected into Escherichia coli BL21/DE3 for protein expression. The recombinant CSPBP was purified using a glutathione sepharose 4B column, and the glutathione S-transferase (GST) tag was removed using a precision protease according to the manufacturer's protocol. To generate polyclonal antisera, CSPBP (without the GST tag) produced in E. coli was emulsified in complete Freund's adjuvant and injected into groups of 4–5 mice (5 μg/mice). Mice were boosted twice at 2-week intervals with the same dose of antigen in incomplete Freund's adjuvant, and sera were collected 2 week after the last boost.
Indirect Immunofluorescence Assay (IFA)
A. gambiae salivary glands were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS), blocked with 5% fetal bovine serum in PBS, sequentially labeled with mouse anti-CSPBP (specific or preimmune serum; dilution, 1:200) and goat anti-mouse immunoglobulin G (IgG) conjugated to Alexa 555, and counterstained with 0.01% TOPRO or ConA conjugated with Alexa 633 (blue). Fixed salivary glands were incubated with biotinylated CSP or an unrelated biotinylated protein (OspC; 2 μg/mL), washed, and incubated with streptavidin conjugated with Alexa Flour-488. For the peptide competition assays, 2 peptides were incubated with fixed salivary gland at different concentrations, prior to incubation with biotinylated CSP (Peptide_ Plasmodium falciparum: KPKHKKLKQPGDGNPDPNAN; Peptide_P. berghei: IERNNKLKQPPPPPNPNDPP). The remainder of the procedure is the same as above. Fluorescence images were obtained with a Zeiss 510 confocal laser-scanning microscope, using identical excitation and detection settings.
Enzyme-Linked Immunosorbent Assay (ELISA) to Assess Binding of CSPBP Antibody to Salivary Gland Proteins
A. gambiae females (5–6 days after emergence) were fed 200 μL of a 1:3 dilution (vol/vol) of CSPBP antibody in PBS and 1 mM adenosine triphosphate through a membrane feeder. Unfed mosquitoes were removed and used as controls. Previous studies have shown that fed antibodies reach maximum hemolymph titers 3 hours after feeding [18]. A pair of whole, intact salivary glands was dissected from each of 15 mosquitoes 3 hours after feeding and extracted by 10 freeze-thaw cycles, followed by centrifugation (Brinkman) at 11,000 g for 2 minutes. The pellet was resuspended in 400 μL of carbonate buffer (4 mM Na2CO3/NaHCO3; pH 9.6), vortexed thoroughly, and used to coat ELISA plate wells in duplicate for each serial dilution. After blocking with 5% bovine serum albumin in PBS/Tween-20, 10 μL of a 1:1000 dilution of horseradish peroxidase–conjugated goat anti-mouse antibody was added (37°C for 1 hour) and developed with 100 μL of TMB. The plate was read with an ELISA reader at 405 nm.
In Vivo Sporozoite Invasion–Blocking Assay
A. gambiae females were allowed to feed for 30 minutes on an anesthetized Swiss Webster mouse infected with P. berghei. Fed mosquitoes were kept at 21°C and 68% relative humidity with alternating light and dark cycles. A total of 4–8 days after infection, 20 mosquito guts were dissected to determine the oocyst infection rates. On day 11 after feeding on an infected blood meal, mosquitoes were randomly divided into 3 cages and fed CSPBP antibodies through a membrane-feeder apparatus in a double-blinded fashion. Salivary glands from individual mosquitoes were removed 3 and 7 days after feeding. The parasite load in salivary glands was determined by measuring Pb18s copies, using qPCR.
Statistical Analysis
All P values were determined with Prism 5.0 software (GraphPad Software) as indicated. A P value of < .05 was considered statistically significant.
RESULTS
A. gambiae CSPBP Interacts With Plasmodium CSP
To identify mosquito protein(s) that interact with Plasmodium falciparum CSP (PfCSP), an A. gambiae cDNA yeast surface display library was probed with biotinylated PfCSP (Supplementary Materials). Magnetic-activated cell sorting was then performed to enrich for clones expressing proteins interacting with PfCSP. One of the candidate genes showed significant similarity to A. gambiae AGAP006649, which encodes a 661–amino acid protein, by in silico analysis in VectorBase (available at: http://vectorbase.org). The protein was named CSPBP. While an N-terminal signal peptide was not evident on SignalP3.0 analysis, nonclassic signal peptide cleavage sites were identified using SecretomeP 2.0. CSPBP was rich in glutamine (43 of 661 amino acids), which may be involved in protein-protein interactions via hydrogen bonds. CSPBP also has 2 coiled-coil domains that often mediate protein-protein interactions (Supplementary Figure 1).
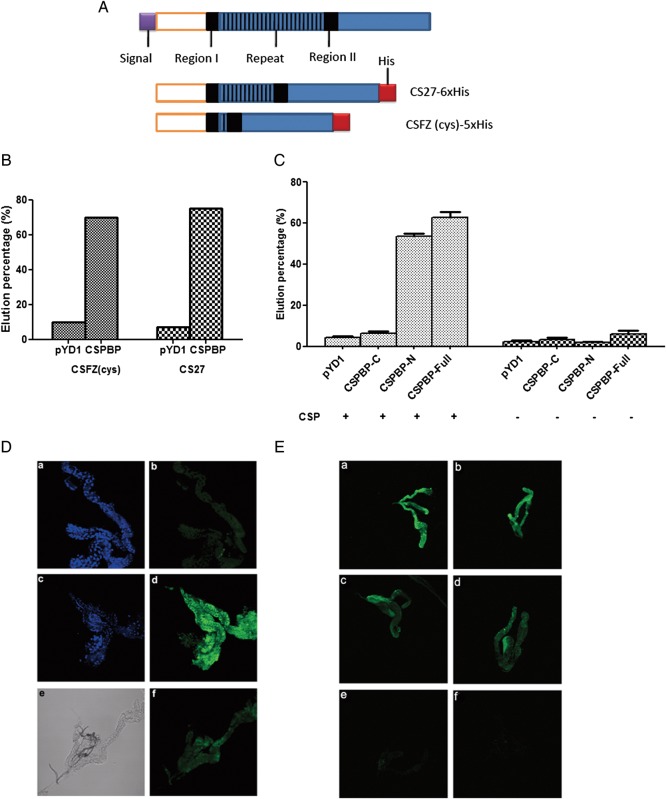
To further assess the specific interaction between CSPBP and PfCSP, the sequenced plasmid containing the CSPBP fragment or an empty vector (pYD1) was introduced into competent yeast cells (EBY100) and probed with 2 forms of PfCSP: the PfCSP containing >1 repeat region (CS27) and a fragment of PfCSP (CSFZ) including 1 repeat region (Figure 1A). PfCSP bound to CSPBP-expressing yeast cells but not to empty vector–expressing cells, with 71% and 65% of yeast cells with CSPBP binding to biotinylated CS27 and CSFZ, respectively, compared with 8.5% and 6.2% of the control cells (Figure 1B). These data also suggest that the repeat region of PfCSP does not significantly contribute to this interaction. We constructed 3 yeast clones expressing the full-length, N-terminal, or C-terminal portions of CSPBP. Yeast containing the full-length protein and the N-terminal fragments of CSPBP but not the C-terminal region bound to PfCSP (Figure 1C).
Figure 1.
In vitro and in vivo protein interactions. A, Schematic diagram of circumsporozoite protein (CSP) and recombinant plasmids with different forms. TSR at the C-terminus is denoted by blue, the conserved region is denoted by black, the species-specific repeats are denoted by black stripes, and the signal peptide is denoted by purple. CS27 is a bacterial expression construct of synthetic Plasmodium falciparum CSP (PfCSP) domains with multiple CSP repeats. CSFZ is a bacterial expression construct that expresses 1 repeat of CSP. B, Yeast cells expressing CSPBP interact with 2 forms of CSP (as in A). Yeast cells expressing CSPBP were enriched through biotinylated CSFZ or CS27. The interaction was assessed by examining the percentage of cells in the eluate. CSFZ and CS27 can enrich equally yeast cells expressing CSPBP. C, The CSPBP N-terminus is important for PfCSP binding. Plasmids containing CSPBP or the empty vector pYD1 were introduced into competent yeast cells (EYB100). Both kinds of yeast cells were grown in SDCAA, induced in SGCAA, and enriched through biotinylated PfCSP or not, and enriched cells were eluted from the magnetic-activated cell sorting column for quantification. Protein interactions were assessed by examining the percentage of cells in the eluate. Both the full-length and N-terminal CSPBPs bind to PfCSP, while the C-terminal fragment does not. D, PfCSP binds to salivary gland and can be blocked by CSPBP antibody. Fixed salivary glands were first incubated with (f) or without (b and d) CSPBP antibody, followed by incubation with 50 nM of biotinylated bovine serum albumin (BSA; b) or biotinylated PfCSP (d and f). Protein binding was detected by incubation with fluorescein isothiocyanate (FITC)–labeled streptavidin. The nuclei were stained with TOPRO3 as shown in a and c. A different interference contrast image of the same gland (f) is shown in e. E, Peptides compete with PfCSP for binding to salivary glands. Fixed salivary glands were first incubated with buffer (control) (a and b), 5 (c and d) or 50 μM (e and f) of nonbiotinylated peptide from P. falciparum (c and e), or P. berghei (d and f), followed by incubation with 50 nM of recombinant PfCSP. PfCSP binding was detected by incubation with FITC-labeled streptavidin. Note that both peptides effectively competed with PfCSP for binding to salivary glands.
We explored this relationship in vivo by incubating recombinant PfCSP with salivary glands. PfCSP bound to the salivary gland surface, and this interaction could be blocked by CSPBP antibody (Figure 1D). We also investigated whether this interaction could be competitively inhibited with the peptide from P. berghei or P. falciparum. As seen in Figure 1E, both peptides competed in a dose-dependent manner with CSP for binding to salivary glands. At 5 μM, only partial inhibition was observed, whereas higher concentrations effectively prevented PfCSP binding. These results suggest that the peptides and PfCSP compete for the same site on the salivary gland surface and that this is most likely CSPBP.
Silencing CSPBP Impairs Sporozoite Invasion of Salivary Glands
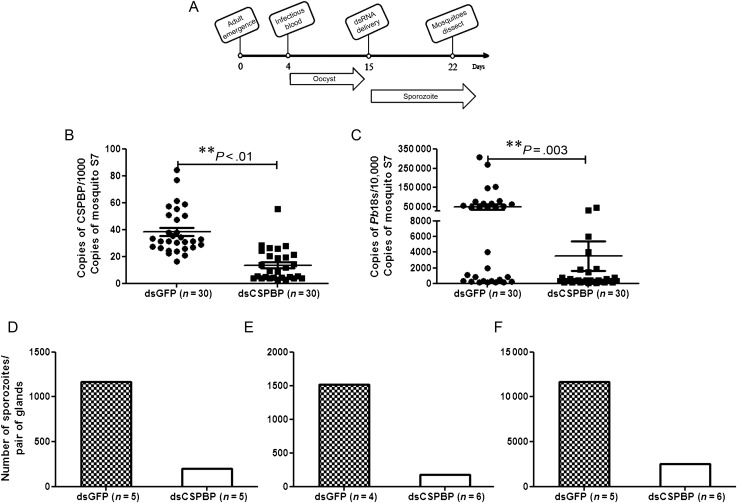
To analyze the potential role of CSPBP on Plasmodium infection of mosquito salivary glands, CSPBP-deficient A. gambiae were generated by RNAi, and P. berghei was used as the model organism because of the ready availability of a murine model for subsequent studies. An experimental timeline for RNAi-mediated repression of CSPBP in A. gambiae salivary glands and its effect on P. berghei sporozoite invasion is outlined in Figure 2A. To detect specific effects on sporozoite development and salivary gland invasion, independent from the effects on oocyst development, CSPBP and GFP dsRNAs were injected into the hemocoel 11 days after the infectious blood meal, at which time oocysts had matured and sporozoites were ready to be released and invade the salivary glands. The silencing of CSPBP was confirmed by qRT-PCR (Figure 2B). Knockdown of gene expression in mosquito salivary glands is technically challenging [19]; however, by using 1000 ng of dsRNA, we observed significantly decreased transcript levels, even 7 days after the dsRNA injection. Salivary gland–associated sporozoite levels in CSPBP-deficient mosquitoes were reduced (Figure 2C). To assess the level of surface-bound and fully internalized sporozoites in CSPBP-deficient mosquitoes, we measured the proportion of salivary gland–associated sporozoites that actually invaded the salivary glands. In 3 separate experiments with CSPBP dsRNA– and GFP dsRNA–injected mosquitoes, salivary glands of mosquitoes obtained on day 18 after feeding were dissected, treated with trypsin to remove surface-bound sporozoites, washed, and ruptured in a ground-fitting homogenizer to free internalized sporozoites. In GFP dsRNA–treated mosquitoes, the proportion of the total salivary gland–associated sporozoites released from within the glands was 83%, confirming the intracellular location of the vast majority of the sporozoites. In contrast, only 25% of the salivary gland–associated sporozoites were released from within the glands in CSPBP-deficient mosquitoes. This indicates that the sporozoites poorly invaded, if at all, the salivary glands of mosquitoes in which CSPBP expression had been reduced. We also observed a 4–5-fold decrease in the number of sporozoites within infected salivary glands on day 18 after the infectious blood meal in 3 independent experiments (Figure 2D–F). This confirmed the qRT-PCR data shown in Figure 2C. Therefore, CSPBP is necessary for efficient salivary gland colonization by P. berghei.
Figure 2.
RNA interference (RNAi)–mediated knockdown of circumsporozoite protein–binding protein (CSPBP) in salivary glands affects Plasmodium berghei sporozoite invasion. A, Experimental time line for RNAi experiments shown in panels B–D. Each time point represents steps in the experimental process and indicates the age of the mosquito at the time of manipulation, the point of delivery of the P. berghei–infected blood meal, and the point of salivary gland harvest, which is 7 days after double-stranded RNA (dsRNA) injection (day 18 after the infectious blood meal). The periods of oocyst development on the mosquito midgut and sporozoites escaping the ruptured oocyst and beginning invasion of the salivary glands are indicated by the arrows. Note that some oocysts will rupture earlier given the asynchronous nature of oocyst development in the mosquito. B, RNAi-mediated knockdown of CSPBP in salivary glands, measured by quantitative reverse-transcription polymerase chain reaction (qRT-PCR). Expression of CSPBP was analyzed by qRT-PCR in the salivary glands 7 days after injection of CSPBP dsRNA (dsCSPBP) or control green fluorescent protein (GFP) dsRNA (dsGFP). C, CSPBP dsRNA reduced sporozoite invasion of salivary glands. P. berghei–infected mosquitoes were injected with dsCSPBP or dsGFP. Mosquito salivary glands were removed from both dsRNA-treated groups 7 days after injection. The P. berghei load in the mosquito salivary glands was quantified by qRT-PCR. Each dot represents 1 mosquito. Data are presented as means ± standard errors of the mean, and P values were analyzed by the Mann–Whitney U test. A P value of < .05 was considered statistically significant. Three independent experiments yielded similar results. D–F, The average sporozoite numbers within salivary glands after infection by P. berghei of mosquitoes treated with dsCSPBP and dsGFP. The salivary glands were treated with trypsin to remove surface-associated sporozoites before counting. Each figure is representative of an independent experiment.
In Vivo Accessibility of CSPBP Antibody to Salivary Glands and Inhibition of Sporozoite Invasion
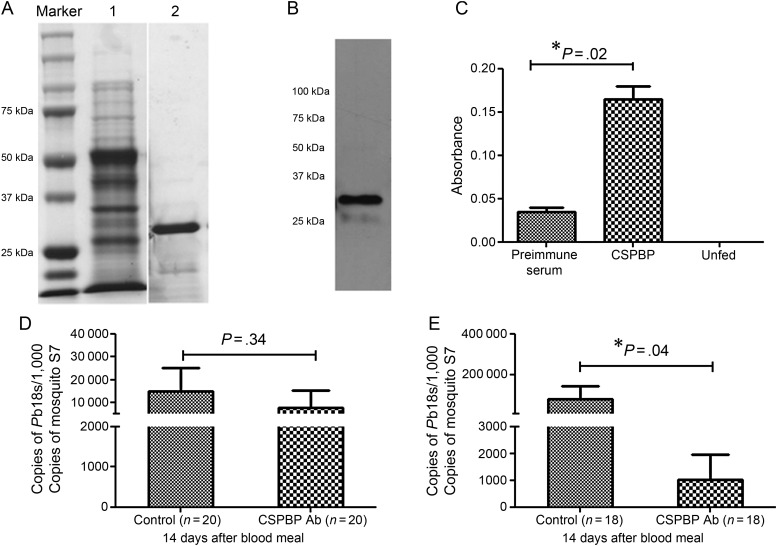
Antibody against the N-terminus of the CSPBP protein was generated in mice (Figure 3A and 3B). An ELISA was used to demonstrate the binding of blood-fed CSPBP antibody to salivary glands in female A. gambiae mosquitoes (6 days after emergence). As shown in Figure 3C, much higher binding was detected for the CSPBP antibody group than for the control (ie, preimmune serum) group. These results demonstrate that the fed CSPBP antibody can reach and bind to salivary glands via hemolymph circulation after traversing through the gut.
Figure 3.
Circumsporozoite protein–binding protein (CSPBP) antibody can inhibit sporozoite infection of salivary gland. A, Coomassie blue staining of expression and purified recombinant fragments of CSPBP on sodium dodecyl sulfate 12% polyacrylamide gel. Lane 1, Whole pellet of bacteria. Lane 2, Purified recombinant protein without the glutathione S-transferase tag. B, CSPBP antiserum recognizes recombinant CSPBP generated in Escherichia coli. C, In vivo binding of fed CSPBP antibodies to salivary glands. Female Anopheles gambiae were fed CSPBP antibody or preimmune serum through a membrane feeder, and salivary gland–bound antibodies were detected by enzyme-linked immunosorbent assay. The graph shows the average absorbance of duplicates with 3 salivary gland equivalents per well. D and E, In vivo blocking of sporozoite infection of salivary glands. The graphs show that the mosquitoes fed CSPBP antibody displayed a 25% and 90% reduction (P = .04) in the parasite load in infected salivary glands at 14 (D) and 18 (E) days after their blood meal, respectively. Data are presented as means ± standard errors of the mean, and P values were analyzed with the Mann–Whitney U test. A P value of < .05 was considered statistically significant. Three independent experiments yielded similar results.
An in vivo sporozoite invasion–blocking assay was developed to determine whether CSPBP antibody blocks the invasion of salivary glands by sporozoites. A. gambiae fed on mice infected with P. berghei. Ten days after the infectious blood meal, 20 mosquitoes were dissected to determine infection rates, and the numbers of oocysts were counted. There was no significant difference in the percentage of mosquitoes infected with oocysts in 3 separate experiments. The mosquitoes were then fed CSPBP antibody on day 11 after infection. One whole, intact pair of salivary glands was dissected and homogenized from each mosquito on days 3 and 7 after antibody feeding, and the parasite load was quantified by qRT-PCR. Infected mosquitoes that were fed the preimmune serum were used as control. The results showed that the mosquitoes that were fed CSPBP antibody displayed a 25% and 90% reduction in the parasite load in infected salivary glands 14 and 18 days, respectively, after the blood meal (Figure 3D and 3E).
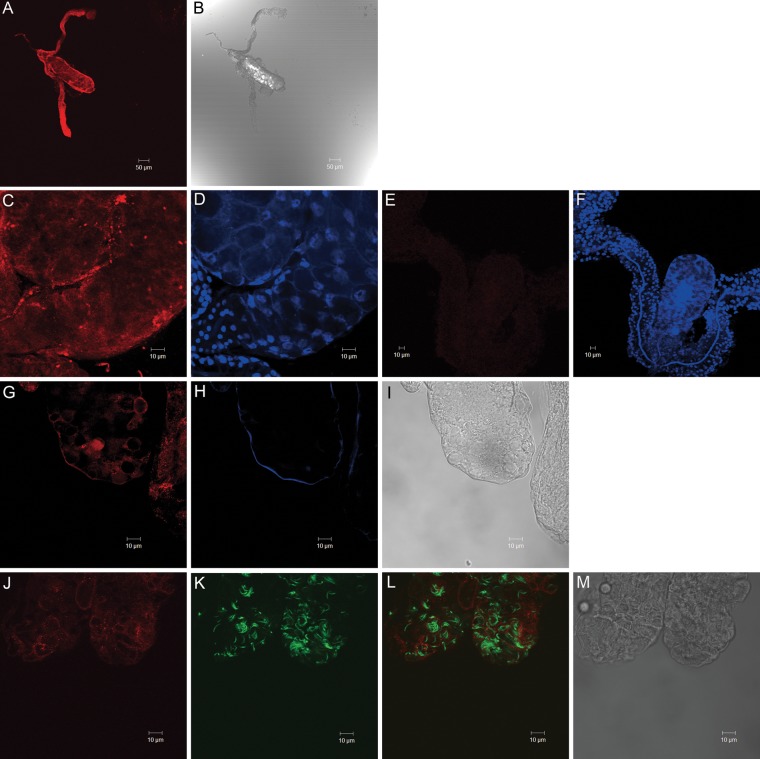
The expression profile of the CSPBP gene was analyzed in the salivary glands, gut, and carcass (whole bodies minus salivary gland and gut) of blood-fed mosquitoes. The expression of CSPBP was detected in all of the organs (Supplementary Figure 2). Anatomical localization of CSPBP was examined by confocal microscopy. CSPBP was expressed in the distal region of the lateral lobes and medial lobes (Figure 4A and 4C). Double IFA labeling with anti-CSPBP and goat anti-mouse-Alexa 555 (Figure 4G), followed by ConA-Alexa 633 (Figure 4H), suggested that the CSPBP label follows the salivary gland basal lamina and the cell membrane of the epithelia. After sporozoite invasion of salivary glands, we could not detect the interaction between CSPBP and sporozoites within salivary glands (Figure 4L). Overall, the microscopic images indicated that at least a portion of CSPBP was exposed on the hemocoel-facing surface of the salivary glands and that the interaction was only on the salivary gland surface and not within the salivary glands.
Figure 4.
Circumsporozoite protein–binding protein (CSPBP) immunolocalization in Anopheles gambiae salivary glands. A, Immunostaining of salivary glands using CSPBP antibody. CSPBP is expressed in the distal region of the lateral and medial lobes. B, Differential interference contrast images. C and D, Confocal microscopy of salivary gland tissues probed with CSPBP antisera. E and F, Murine preimmune sera served as a control. Nuclei were stained with TOPRO (D and F). G, CSPBP antibody labeled the salivary gland basal lamina and the membrane of the epithelial cells. H, Con A stained the basal lamina of salivary gland lobule. I, The same lobe of the salivary gland in the differential interference contrast. J–L, Salivary glands from Plasmodium berghei–infected mosquitoes were probed with CSPBP antibody (J). Sporozoites expressing green fluorescent protein are shown in green (K). Colocalization (yellow) was not noted in panel L. M, A differential interference contrast image of the same salivary gland. Images are representative of 10 independent experiments.
DISCUSSION
Salivary gland invasion is an essential step of the Plasmodium life cycle in its mosquito vector. Circumstantial evidence suggests that this process is mediated by specific vector-pathogen ligand interactions. It has previously been reported that CSP binds to salivary glands and that a peptide from the N-terminal portion of the protein could inhibit binding of the entire protein to salivary glands [8]. These data suggest that CSP is involved in sporozoite invasion of salivary glands; however, the lack of in vitro invasion assays for oocyst sporozoites has made it difficult to test this hypothesis. Here, we report that sporozoites bind to a mosquito protein via CSP, therefore facilitating invasion of the salivary glands. We performed a yeast surface display screening and identified and characterized a candidate mosquito salivary protein, AGAP006649 (CSPBP), which interacts with the Plasmodium CSP.
The yeast clone expressing CSPBP bound to 2 forms of PfCSP: CS27, with multiple repeat regions, and CSFZ (Cys), with only 1 repeat region. This indicated that multiple repeat regions are unlikely to contain the sporozoite species–specific salivary gland ligand. This finding was consistent with a report that the repeat region is not responsible for salivary gland invasion [20]. The N-terminal peptide that interferes with PfCSP binding to and sporozoite invasion of salivary glands is from the PfCSP and includes the highly conserved region I (KLKQP), which is present in different species of Plasmodium. This suggests that P. falciparum and P. berghei both interact with CSPBP in a similar fashion, which is consistent with our initial screening results using PfCSP, our in vivo studies using P. berghei, and the binding competition studies using peptides from both Plasmodium species.
Sequence analysis reveals that CSPBP has a relatively high abundance of glutamine residues. CSPBP also has 2 coiled-coil domains that often mediate protein-protein interactions. We showed that yeast cells expressing CSPBP N-terminal fragments, which contain the coiled-coil domains, had high affinity for recombinant PfCSP, suggesting that these regions perform this function.
It is likely that CSPBP and CSP are not the only molecules that are important for salivary gland invasion by midgut sporozoites [21]. In addition to Plasmodium CSP, TRAP [22] and another micronemal protein, MAEBL [11], participate in sporozoite invasion of the salivary gland. Mosquito saglin interacts with TRAP [9], and a salivary gland ligand for MAEBL has not yet been identified. In addition to saglin, SGS1 is a potential candidate receptor for Plasmodium organisms on the salivary gland surface [12, 23], and it is interesting that both are also present in the saliva. Similar to CSPBP, Anopheles SGS also has a nonclassic secretion sequence [23]. In contrast, saglin has a signal peptide [14]. Recently, 2 additional A. gambiae proteins, named epithelial serine protease and Plasmodium responsive salivary 1, have been suggested to play a role in both oocyst development and sporozoite invasion of salivary glands [24, 25]. A conserved domain in all 5 proteins is not apparent. CSPBP and saglin, however, have a coiled-coil domain in their N-terminal regions, which may contribute to the interaction. Whether all of these salivary gland proteins act in a complex interdependent manner or separately to facilitate invasion, as well as the relative contribution of each molecule to the overall process, remain to be determined.
Sporozoites can differentiate among the diverse mosquito organs associated with the hemocoel. Because CSPBP is not expressed exclusively in the salivary glands, this protein is not likely involved in this process. CSPBP, however, may facilitate infection of the salivary glands in conjunction with other sporozoite proteins, perhaps by enhancing the affinity of attachment along with other molecules. CSPBP is expressed throughout the salivary glands (Figure 4A), including the proximal lateral lobes and the salivary duct, as well as the regions that sporozoites invade. Anopheles SGSs are also present in the acinar cells of the distal lateral lobes and in the salivary ducts of the proximal lobes [23]. This suggests that CSPBP and SGSs may also facilitate the ability of sporozoites to traverse acinar cells and survive in the salivary duct.
This work demonstrates an important role for the interaction between Anopheles CSPBP and Plasmodium CSP in sporozoite infection of the mosquito salivary gland and that interfering with this relationship can interrupt the life cycle of the malaria parasite. A greater understanding of this and similar pathways may lead to new transmission-blocking vaccines that may interfere with the spread of malaria.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Eva Shyqyriu-Gega, Ying Yang, and Jingyi Pan for assistance. The following reagents were obtained through the MR4 as part of the BEI Resources Repository, National Institute of Allergy and Infectious Diseases, National Institutes of Health: P. falciparum pDS56-32/RBSII-CSFZ(Cys)-5XHis, MRA-271, and P. falciparum pDS56-32/RBSII-CS27IVC-6XHis, MRA-272, deposited by P. Sinnis.
J. W., Y. Z., and E. F. conceived and designed the experiments. J. W., Y. Z., Y. O. Z., M. L., S. M. D., and N. A. performed the experiments. J. W., Y. Z., and L. Z. analyzed the data. J. W. and E. F. wrote the manuscript.
Financial support. This work was supported by the Howard Hughes Medical Institute (to E. F.).
Potential conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Murray CJ, Rosenfeld LC, Lim SS, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–31. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh A, Edwards MJ, Jacobs-Lorena M. The journey of the malaria parasite in the mosquito: hopes for the new century. Parasitol Today (Regul Ed) 2000;16:196–201. doi: 10.1016/s0169-4758(99)01626-9. [DOI] [PubMed] [Google Scholar]
- 3.Pimenta PF, Touray M, Miller L. The journey of malaria sporozoites in the mosquito salivary gland. J Eukaryot Microbiol. 1994;41:608–24. doi: 10.1111/j.1550-7408.1994.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg R. Inability of Plasmodium knowlesi sporozoites to invade Anopheles freeborni salivary glands. Am J Trop Med Hyg. 1985;34:687–91. doi: 10.4269/ajtmh.1985.34.687. [DOI] [PubMed] [Google Scholar]
- 5.Barreau C, Touray M, Pimenta PF, Miller LH, Vernick KD. Plasmodium gallinaceum: sporozoite invasion of Aedes aegypti salivary glands is inhibited by anti-gland antibodies and by lectins. Exp Parasitol. 1995;8:332–43. doi: 10.1006/expr.1995.1124. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh AK, Jacobs-Lorena M. Plasmodium sporozoite invasion of the mosquito salivary gland. Curr Opin Microbiol. 2009;12:394–400. doi: 10.1016/j.mib.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ménard R, Sultan AA, Cortes C, et al. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature. 1997;385:336–40. doi: 10.1038/385336a0. [DOI] [PubMed] [Google Scholar]
- 8.Sidjanski SP, Vanderberg JP, Sinnis P. Anopheles stephensi salivary glands bear receptors for region I of the circumsporozoite protein of Plasmodium falciparum. Mol Biochem Parasitol. 1997;90:33–41. doi: 10.1016/s0166-6851(97)00124-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh AK, Devenport M, Jethwaney D, et al. Malaria parasite invasion of the mosquito salivary gland requires interaction between the Plasmodium TRAP and the Anopheles saglin proteins. PLoS Pathog. 2009;5:e1000265. doi: 10.1371/journal.ppat.1000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson J, Fernandez-Reyes D, Sharling L, et al. Plasmodium cysteine repeat modular proteins 1-4: complex proteins with roles throughout the malaria parasite life cycle. Cell Microbiol. 2007;9:1466–80. doi: 10.1111/j.1462-5822.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- 11.Kariu T, Yuda M, Yano K, Chinzei Y. MAEBL is essential for malarial sporozoite infection of the mosquito salivary gland. J Exp Med. 2002;195:1317–23. doi: 10.1084/jem.20011876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korochkina S, Barreau C, Pradel G, et al. A mosquito-specific protein family includes candidate receptors for malaria sporozoite invasion of salivary glands. Cell Microbiol. 2006;8:163–75. doi: 10.1111/j.1462-5822.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- 13.Brennan JD, Kent M, Dhar R, Fujioka H, Kumar N. Anopheles gambiae salivary gland proteins as putative targets for blocking transmission of malaria parasites. Proc Natl Acad Sci U S A. 2000;97:13859–64. doi: 10.1073/pnas.250472597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okulate MA, Kalume DE, Reddy R, et al. Identification and molecular characterization of a novel protein Saglin as a target of monoclonal antibodies affecting salivary gland infectivity of Plasmodium sporozoites. Insect Mol Biol. 2007;16:711–22. doi: 10.1111/j.1365-2583.2007.00765.x. [DOI] [PubMed] [Google Scholar]
- 15.Rosinski-Chupin I, Briolay J, Brouilly P, et al. SAGE analysis of mosquito salivary gland transcriptomes during Plasmodium invasion. Cell Microbiol. 2007;9:708–24. doi: 10.1111/j.1462-5822.2006.00822.x. [DOI] [PubMed] [Google Scholar]
- 16.Franke-Fayard B, Trueman H, Ramesar J, et al. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol Biochem Parasitol. 2004;137:23–33. doi: 10.1016/j.molbiopara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Blandin S, Moita LF, Kocher T, Wilm M, Kafatos FC, Levashina EA. Reverse genetics in the mosquito Anopheles gambiae: targeted disruption of the Defensin gene. EMBO Rep. 2002;3:852–6. doi: 10.1093/embo-reports/kvf180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaughan JA, Wirtz RA, do Rosario VE, Azad AF. Quantitation of antisporozoite immunoglobulins in the hemolymph of Anopheles stephensi after bloodfeeding. Am J Trop Med Hyg. 1990;42:10–16. doi: 10.4269/ajtmh.1990.42.10. [DOI] [PubMed] [Google Scholar]
- 19.Boisson B, Jacques JC, Choumet V, et al. Gene silencing in mosquito salivary glands by RNAi. FEBS Lett. 2006;580:1988–92. doi: 10.1016/j.febslet.2006.02.069. [DOI] [PubMed] [Google Scholar]
- 20.Aldrich C, Magini A, Emiliani C, et al. Roles of the Amino Terminal Region and Repeat Region of the Plasmodium berghei Circumsporozoite Protein in Parasite Infectivity. PLoS One. 2012;7:e32524. doi: 10.1371/journal.pone.0032524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hegge S, Munter S, Steinbuchel M, et al. Multistep adhesion of Plasmodium sporozoites. FASEB J. 2010;24:2222–34. doi: 10.1096/fj.09-148700. [DOI] [PubMed] [Google Scholar]
- 22.Sultan AA, Thathy V, Frevert U, et al. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90:511–22. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- 23.King JG, Vernick KD, Hillyer JF. Members of the salivary gland surface protein (SGS) family are major immunogenic components of mosquito saliva. J Biol Chem. 2011;286:40824–34. doi: 10.1074/jbc.M111.280552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodrigues J, Oliveira GA, Kotsyfakis M, et al. An epithelial serine protease, AgESP, is required for Plasmodium invasion in the mosquito Anopheles gambiae. PLoS One. 2012;7:e35210. doi: 10.1371/journal.pone.0035210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chertemps T, Mitri C, Perrot S, et al. Anopheles gambiae PRS1 modulates Plasmodium development at both midgut and salivary gland steps. PLoS One. 2010;5:e11538. doi: 10.1371/journal.pone.0011538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.