Abstract
Endometrial stromal sarcoma (ESS) is a rare uterine sarcoma. Compared with other uterine malignancies, it occurs at an earlier age (42–58 years) and about 10–25% of the patients are premenopausal. The tumours have an indolent growth, with a tendency for late recurrence. Metastases are rarely detected before the diagnosis of the primary lesion. We report a case of ESS with pulmonary metastasis as a prodromal manifestation.
Background
Endometrial stromal tumours of the uterus are the second most common mesenchymal tumours of the uterus. In the latest WHO classification, endometrial stromal tumours are divided into three categories: (1) endometrial stromal nodule, (2) low-grade endometrial stromal sarcoma (ESS) and (3) undifferentiated endometrial sarcoma (UES).1
In contrast to low-grade ESS, which is composed of cells that morphologically resemble non-neoplastic proliferative-phase endometrial stroma, UES does not resemble endometrial stroma but exhibits marked cytological atypia with nuclear pleomorphism, high mitotic rate and tumour necrosis. In recent years, it has become increasingly clear that this classification does not adequately reflect the morphological heterogeneity that occurs in malignant stromal tumours. For example, some uterine sarcomas can display cytological and immunohistochemical (IHC) features that are intermediate between classic low-grade ESS and UES, which have been classified as UES with nuclear uniformity (UES-U).2 In keeping with this varied histological appearance, it has been shown that ESS is genetically heterogeneous as well.3 Here we report a case of ESS with pulmonary metastasis as a prodromal manifestation. In addition, the tumour cells were CD10 negative and showed some sex cord-like differentiation.
Case presentation
A 50-year-old woman was admitted to our pulmonology department with dyspnoea, cough and blood-tinged sputum, which persisted for 3 months. She had a history of breast carcinoma and left-sided mastectomy was performed 4 years ago, with subsequent chemoradiotherapy.
Investigations
Examination of the chest revealed fine crepitations in both lungs. Abdominal examination showed hypogastric pain and a hard-to-define mass. Gynaecological examination revealed a hard-to-delimitate uterus and a painful posterior vaginal wall. A chest X-ray showed a mass in the left lower lobe. CT of the lung revealed two masses in the left lower lobe (figure 1). Bronchoscopy revealed no endobronchial lesions and the bronchial washings did not show cytological or microbiological abnormalities. Considering the patient's history of mastectomy, the first clinical impression was metastatic breast carcinoma. Vaginal ultrasound was performed, which revealed uterine enlargement with an image of a nodular interstitial formation measuring 60×50 mm. Pelvic CT revealed a mass in the fundal area of the uterus (figure 2). Laboratory findings showedcancer antigen 125 (CA-125) 78 IU/mL (normal value up to 35 IU/ml). A CT-guided biopsy of the lung was performed. Microscopic examination revealed neoplastic round to oval cellswith scant cytoplasm and round to oval or angular nuclei. Tumour cells were arranged in a diffuse pattern and stained positive for CD99 and vimentin but stained negative for CK7, CK20, CKAE1/AE3, CD45, ER and PR. According to the morphological and IHC findings, metastasis of a sex cord-stromal tumour was considered in the differential diagnosis. On the basis of these findings, we performed an exploratory laparotomy followed by a tumour debulking procedure. The surgical procedure consisted of a hysterectomy with bilateral salpingoophorectomy, an omentum biopsy and cytology. Both ovaries were normal in size, but the uterus was enlarged and tumoral. The uterus weighed 350 g and measured 11 cm in height; its thickness varied from 1 to 5 cm and in its fundal area, there was a 6.5 cm hard mass with ill-defined margins. The sigmoid colon contained a serosal nodule about 1 cm in diameter that was excised. The appendix, liver surface and diaphragm were normal. Microscopically, the mentioned mass showed a high cellular neoplasm with a solid pattern. The pattern of growth was mainly expansive, infiltrating the adjacent myometrium with vascular invasion (figure 3). The tumour cells were oval to round with a high nucleus/cytoplasm ratio. The nucleus showed slight atypia on low power. But on high-power examination, the nuclei exhibited some membrane irregularity with an angulated contour. There were 25 mitoses/10 HPF (figure 4). IHC stains of the fundal mass specimen revealed positive results with vimentin, α-smooth muscle actin (focally) and CD99, but immunoreactivity to CD10, ER, PR, calretinin, EMA, CK 7, CK20, CKAE1/AE3 andhuman melanoma black 45 (HMB45)was not found (figures 5 and 6). h-Caldesmon is not available in Iran, but the tumour cells stained negative for desmin (figure 7). It should be mentioned that focal reactivity to α-smooth muscle actin may be related to the entrapped myometrial smooth muscles that were immunoreactive for this marker. In addition, the neoplastic cells showed nuclear cyclin D1 staining (figure 8).
Figure 1.
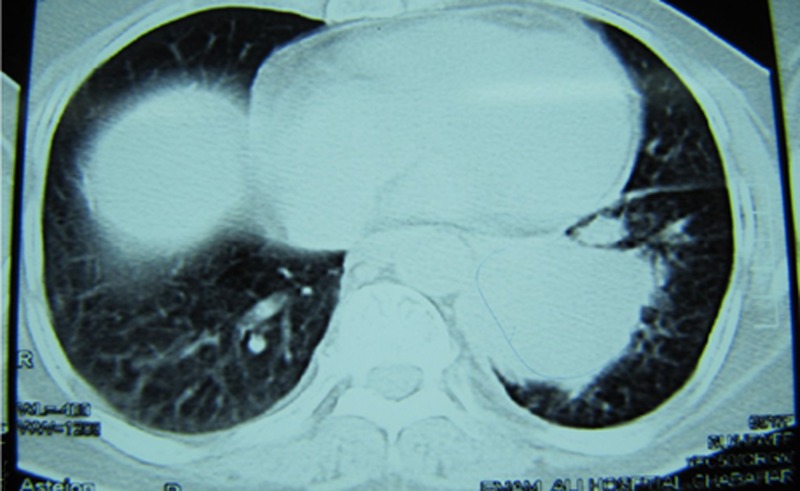
CT scan of the lung showing two masses in the left lower lobe.
Figure 2.
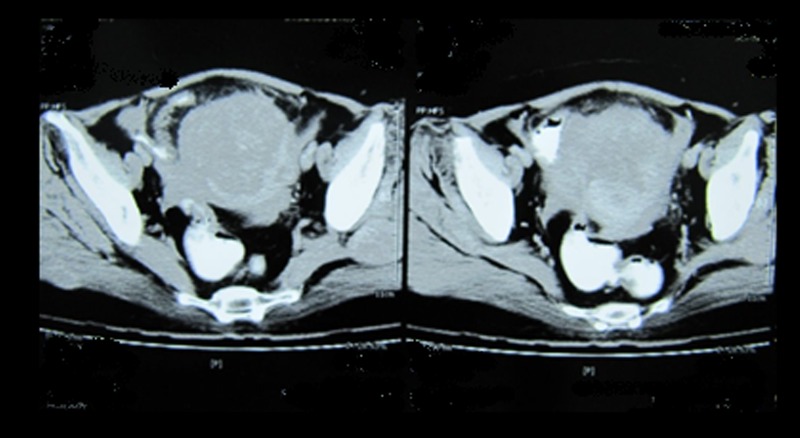
Pelvic CT revealing a mass in the fundal area of the uterus.
Figure 3.
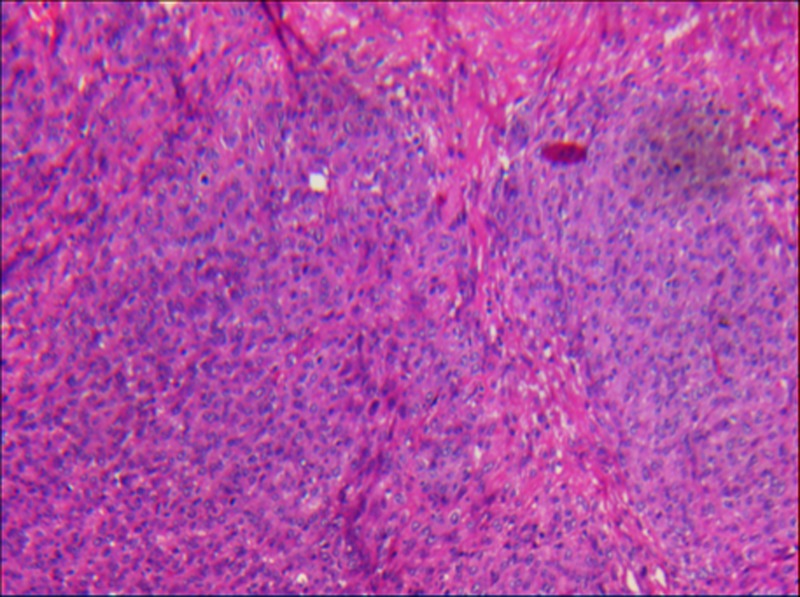
A section showing a high cellular neoplasm with a solid pattern. The pattern of growth was mainly expansive and infiltrating the adjacent myometrium (H&E ×10).
Figure 4.
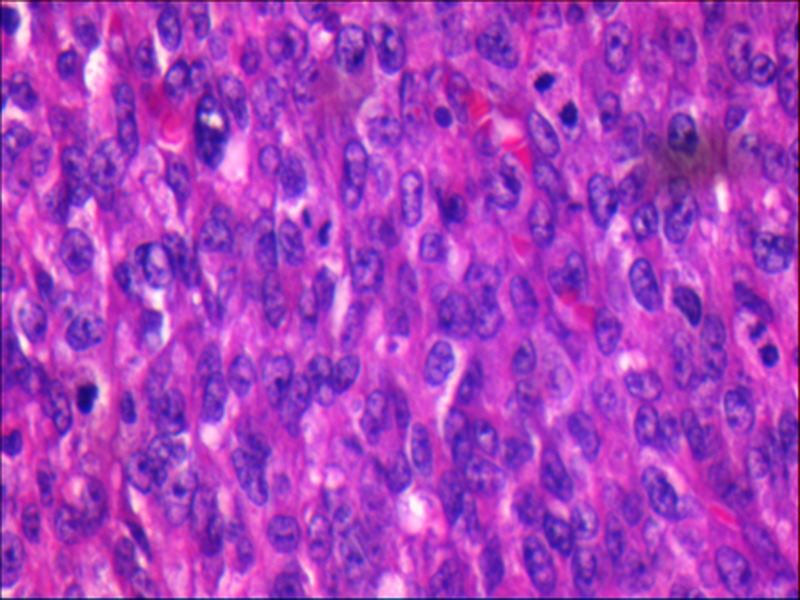
A section showing neoplastic round to oval cells with a scant cytoplasm and the nuclei exhibiting some membrane irregularities with an angulated contour. Mitotic figures were evident (H&E ×40).
Figure 5.
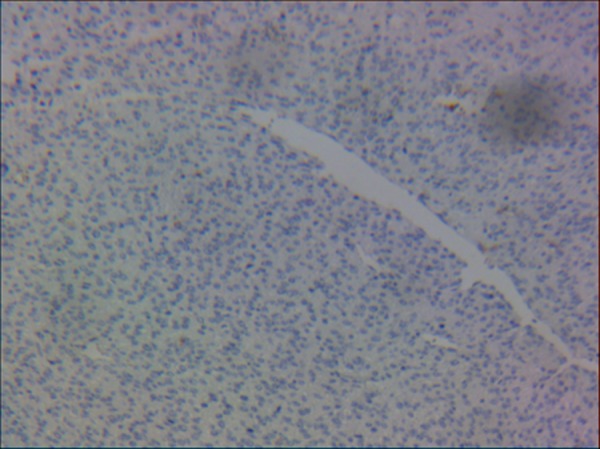
A section revealing that tumour cells were not immunoreactive to CD10 (IHC stain ×10).
Figure 6.
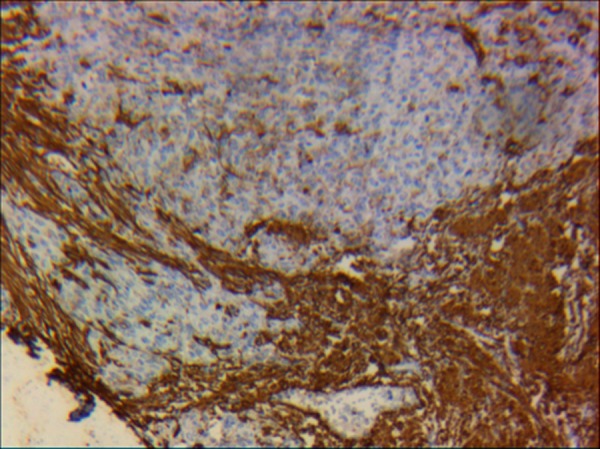
A section revealing that tumour cells were focally immunoreactive to α-smooth muscle actin, unlike myometrial smooth muscle cells which were strongly immunoreactive (IHC stain ×10).
Figure 7.
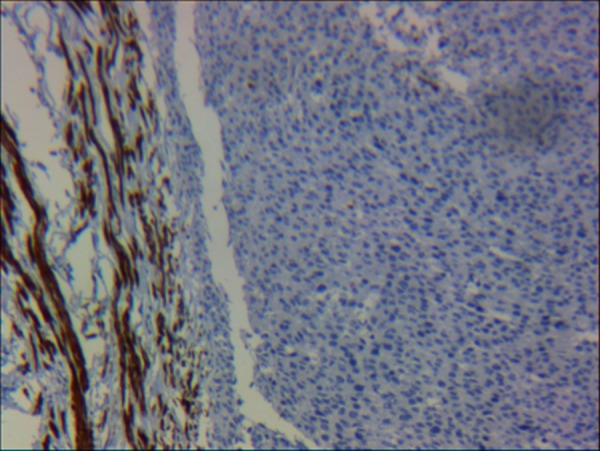
A section showing that neoplastic cells were not immunoreactive to demin, unlike myometrial smooth muscle cells which were strongly immunoreactive (IHC stain ×10).
Figure 8.
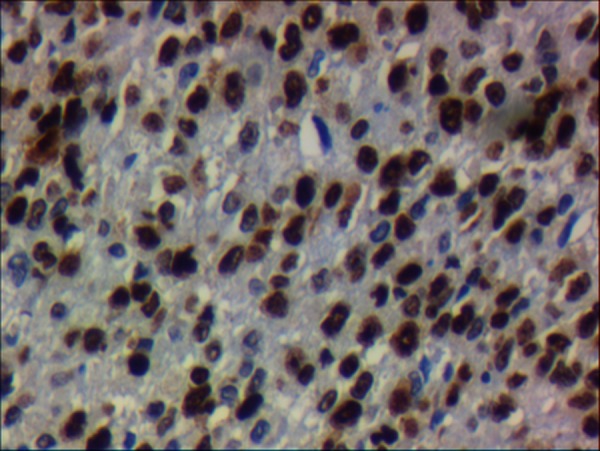
A section showing that neoplastic cells were immunoreactive to cyclin D1 (×40).
Differential diagnosis
Regarding the differential diagnosis, which is mainly with the epithelioid cellular variant of smooth muscle tumours, features that favour a diagnosis of endometrial stromal tumour are multinodular pattern of growth, spiral-type arterioles (some with hyalinised walls), lack or inconspicuousness of large thick-walled vessels and cleft-like spaces. In addition, EESs have extensive permeative growth through the myometrium with vascular invasion. Lack of reactivity for h-caldesmon, desmin and the oxytocin receptor4 is also important. The presented tumour also displayed features of sex cord-like differentiation.
Uterine tumour resembling an ovarian sex cord tumour may enter in this differential diagnosis as ESS may have sex cord-like differentiation. The final diagnosis can only be established with a hysterectomy specimen.
Treatment
The patient was referred to the oncology department for further therapy. But the patient suddenly lost consciousness. An emergency CT scan revealed multiple brain haemorrhagic metastases (figure 9).
Figure 9.
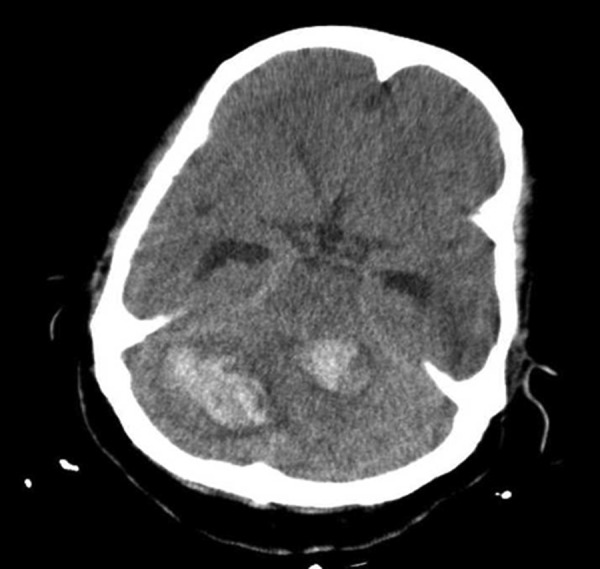
A brain CT scan revealing multiple brain haemorrhagic metastases.
Outcome and follow-up
The patient died due to intracranial haemorrhagic metastases.
Discussion
The first description of ESS was published by Norris and Taylor.5 It accounts for 0.2% of all malignant tumours of the female genital tract.6 The mean age of women with ESS is 42–58 years, while the woman in our case was 50 years old.
The pathogenesis of ESS is unknown, but exposure to tamoxifen, unopposed oestrogens and conditions such as polycystic disease of the ovary has been implicated.7 Our patient had a history of breast carcinoma and she had received tamoxifen. The patients commonly present with abnormal uterine bleeding, pelvic pain and dysmenorrhoea, but as many as 25% of patients are asymptomatic.1 About 30–50% of ESS have extrauterine spread at the time of the diagnosis.8 ESS is rarely present initially at an extrauterine site, most commonly the ovary. The current case did not give any history related to her genital tract and she had been presented due to respiratory symptoms.Common locations of distant metastases are the vagina, vulva, lung, mediastinum, abdomen, bones and ovaries.9 There are small cases of unusual metastatic sites in patients with ESS such as the brain,10 heart,11 stomach12 and sigmoid colon.13 Butwhat distinguishes this case is the fact that pulmonary metastases was the initial presentation of ESS, the patient had a history of breast carcinoma andimmunoreactivity to CD10 was not found. Although CD10 immunoreactivity is a well-known positive predictive marker of ESS, there are exceptional cases when CD10 is negative, namely in fibrous variants.14 To explain the unusual presentation and immunoreactivity, it should be noted thatin recent years it has become increasingly clear that the current classification scheme does not adequately reflect the morphological diversity that can occur in malignant stromal tumours. For example, some uterine sarcomas can display cytological and IHC features that are intermediate between classic low-grade ESS and UES, which have been described in the literature as UES-U. Moreover, other tumours may show areas of UES juxtaposed to low-grade areas. In both instances, however, tumours behave in a more aggressive manner compared with classic low-grade ESS. In addition to high-grade cytological features, ESS can also display variant histological appearances, including smooth muscle, sex cord, glandular and fibrous differentiation.15 According to this concept, a study2 has divided undifferentiated sarcoma into two categories: UES-U and UES with nuclear pleomorphism, and compared their molecular genetic and IHC profiles. They have proposed that UESs showing nuclear regularity may represent an intermediate subcategory of endometrial stromal tumours (formerly classified as high-grade ESSs). In addition, ESS is a genetically heterogeneous group of uterine sarcomas, of which almost half are associated with JAZF1 rearrangement. Lee et al16 recently identified a novel genetic fusion between YWHAE and FAM22A/B in ESS harbouring t10 17 (q22;p13). They described the histological and IHC features for YWHAE-FAM22 ESS. These tumours display high-grade (but non-pleomorphic) round cell histology that is immunophenotypically undifferentiated (CD10, ER and PR negative) and frequently include an admixed low-grade spindle cell component with fibrous/fibromyxoid stroma that is positive for ER, PR and CD10 immunohistochemically. The authors stated that “even though YWHAE-FAM22 ESS exhibits higher-grade nuclear features compared with JAZF1-rearranged ESS, it does not display marked nuclear pleomorphism, which distinguishes it from most UESs.” On the other hand, Chang et al7 have previously described a group of high-grade ESS characterised by nuclei that are more uniform with some irregular nuclear membrane than usual low-grade ESS; this seems similar to what is described for YWHAE-FAM22 ESS. It seems like YWHAE-FAM22 ESS represents a clinically aggressive subtype of ESS, and its distinction from usual low-grade ESS with JAZF1 rearrangement is important to guide clinical management. We were unable to show this genetic fusion in our case but regarding its morphology, IHC findings and especially clinical course, we believe that it is related to this new category. In addition Lee et al17 have shown that cyclin D1 is a sensitive and specific diagnostic immunomarker for YWHAE-FAM22 ESS. It was true in our case. We agree with Mentrikoski et al18 that the correlation of clinical history and imaging studies with histological and IHC findings is essential to the diagnosis of metastatic ESS in the lung. A combination of surgery and adjuvant radiotherapy may improve the treatment outcome.19 Our patient underwent a tumour debunking procedure but died before receiving chemotherapy due to haemorrhagic brain metastases.
Learning points.
Endometrial stromal sarcoma (ESS) is rarely present initially at an extrauterine site metastasis and in these instances diagnostic difficulties may arise.
Recognition of the morphological features, together with the immunoreactivity pattern, is needed in evaluating ESSs that present as extrauterine metastases.
Immunohistochemical results should always be correlated with the histological appearance of the tumour and interpreted accordingly, as ESSs may show areas with different types of differentiation.
In recent years, it has become increasingly clear that the current classification scheme does not adequately reflect the morphological diversity that can occur in malignant stromal tumours.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Oliva E. Pure mesenchymal and mixed Müllerian tumors of the uterus. In: Nucci MR, Oliva E. eds. Gynecology pathology. USA: Elsevier Churchill Livingstone, 2009:293–302 [Google Scholar]
- 2.Kurihara S, Oda Y, Ohishi Y, et al. Endometrial stromal sarcomas and related high-grade sarcomas: immunohistochemical and molecular genetic study of 31 cases. Am J Surg Pathol 2008;2013:1228–38 [DOI] [PubMed] [Google Scholar]
- 3.Sandberg AA. The cytogenetics and molecular biology of endometrial stromal sarcoma. Cytogenet Genome Res 2007;2013:182–9 [DOI] [PubMed] [Google Scholar]
- 4.Rosai J. Rosai and Ackerman's surgical pathology, female reproductive system, uterus-corpus. 10th edn. Vol 2013 Elsevier Mosbey, 2011:1501–3 [Google Scholar]
- 5.Norris HJ, Taylor HB. Mesenchymal tumors of the uterus: I. A clinical and pathological study of 53 endometrial stromal tumors. Cancer 1 1966;2013:755–66 [DOI] [PubMed] [Google Scholar]
- 6.De Fusco PA, Gaffey TA, Malkasian GD, Jr, et al. Endometrial stromal sarcoma: review of Mayo Clinic experience, 1945–1980. Gynecol Oncol 1989;2013:8–14 [DOI] [PubMed] [Google Scholar]
- 7.Chang KL, Crabtree GS, Lim-Tan SK, et al. Primary uterine endometrial stromal neoplasms. A clinicopathologic study of 117 cases. Am J Surg Pathol 1990;2013:415–38 [DOI] [PubMed] [Google Scholar]
- 8.Cohen I. Endometrial pathologies associated with postmenopausal tamoxifen treatment. Gynecol Oncol 2004;2013:256–66 [DOI] [PubMed] [Google Scholar]
- 9.Tavassoli FA, Devilee P. WHO classification of pathology and genetics of tumours of the breast and female genital organs. In: Tavassoli FA, Devilee P. eds. Lyon, France: IARC Press, 2003:233–6 [Google Scholar]
- 10.Merinsky A, Schmidt M, Heidner K, et al. Cerebral metastases of an endometrial stromal sarcoma: report of the first case. Onkologie 2012;2013:272–4 [DOI] [PubMed] [Google Scholar]
- 11.Suzuki S, Yasuhara K, Koyano T, et al. [Tumor thrombectomy for endometrial stromal sarcoma extending into the inferior vena cava and the right atrium from the uterus]. Kyobu Geka 2008;2013:139–42 [PubMed] [Google Scholar]
- 12.You SY, Woo IS, Kim YJ, et al. A case of high-grade endometrial stromal sarcoma with metastasis to the stomach Korean. J Gastrointest Endosc 2010;2013:219–23 [Google Scholar]
- 13.Asada Y, Isomoto H, Akama F, et al. Metastatic low-grade endometrial stromal sarcoma of the sigmoid colon three years after hysterectomy. World J Gastroenterol 2005;2013:2367–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCluggage WG. Immunohistochemistry as a diagnostic aid in cervical pathology. Pathology 2007;2013:97–111 [DOI] [PubMed] [Google Scholar]
- 15.Oliva E, Clement PB, Young RH. Endometrial stromal tumors: an update on a group of tumors with a protean phenotype. Adv Anat Pathol 2000;2013:257–81 [DOI] [PubMed] [Google Scholar]
- 16.Lee C-H, Mariño-Enriquez WA, Ou W, et al. The clinicopathologic features of YWHAE-FAM22 endometrial stromal sarcomas: a histologically high-grade and clinically aggressive tumor. Am J Surg Pathol 2012;2013:641–53 [DOI] [PubMed] [Google Scholar]
- 17.Lee CH, Ali RH, Rouzbahman M, et al. Cyclin D1 as a diagnostic immunomarker for endometrial stromal sarcoma with YWHAE-FAM22 rearrangement. Am J Surg Pathol 2012;2013:1562–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mentrikoski MJ, Zhao C, Zhang J, et al. Metastatic endometrial stromal sarcoma of the lung: importance of immunohistochemical staining, clinical history and imaging studies. Biotech Histochem 2012;2013:35–9 [DOI] [PubMed] [Google Scholar]
- 19.Weitmann HD, Knocke TH, Kucera H, et al. Radiation therapy in the treatment of endometrial stromal sarcoma. Int J Radiat Oncol Biol Phys 2001;2013:739–48 [DOI] [PubMed] [Google Scholar]


