Abstract
Introduction: The large number of patients that require fine needle aspiration biopsy (FNAB) to discriminate malignant from benign thyroid nodules is a practical problem especially in iodine deficient area. To obtain an ultrasound (US) score and for predicting malignant nodules and reduce the number of unnecessary and expensive FNAB. Materials and Methods: A total of 280 thyroid nodules observed from August 2009 to August 2011 that had underwent FNAB were evaluated by US for echogenicity, peripheral halo, microcalcifications and intranodular vascularity. Results: showed that nodules with two ultrasonographic features (US score = 4) were characterized by a 67.9% sensitivity and a 87% specificity for prediction of malignant thyroid nodules. Conclusion: According to our data, we suggest FNAB for nodules with US score ≥ 4. The practical use of this US score may help reduce unnecessary and expensive FNAB especially in iodine-deficient areas.
Keywords: Goiter, nodular, thyroid nodule, thyroid cancer
Introduction
Thyroid nodule is one of the most common clinical problems of the thyroid gland. The Ultrasonographic incidence of thyroid ranges from 10% to 67% [1]. Malignant transformation occurs in 5-10% of thyroid nodules depending on age, gender, history of radiation exposure and a family history of cancer [2].
Fine needle aspiration biopsy (FNAB) of the thyroid now has been accepted as the most reliable diagnostic procedure to reduce the rate of unnecessary thyroid surgery for patient’s thyroid nodules especially when ultrasound guidance is used [3].
The high prevalence of thyroid nodules in iodine-deficient areas is a practical problem because of the large number of patients requiring FNAB to detect malignant nodules. Previous studies have been conducted to identify ultrasonographic criteria’s that can indicate malignancy [4-6].
The aim of our study is to obtain an ultrasound (US) score for predicting malignant nodules and reduce the number of unnecessary and expensive FNAB.
Material and methods
Our study was performed retrospectively and it was approved by the institutional review board. Informed consent was not required. From August 2009 to August 2011, 280 thyroid nodules in 280 consecutive patients underwent US and US-FNAB in our Radiology department and one Radiologist performed all of them.
Only those patients with definite diagnosis according to FNAB report were included in the study. Those nodules that showed no change in one year of follow-up or showed the same cytological results after repeated FNA cytology was considered as benign. Those nodules with Indeterminate, suspicious for malignancy, or inadequate cytological results in FNAB were excluded from study.
Thyroid grey scale and color Doppler US was performed by a Medison Accuvix V 20 scanner (Medison, South Korea) with a 7- to 12-MHz bandwidth transducer. Characterization of ultrasonographic appearance of thyroid nodules was performed before US-FNAB.
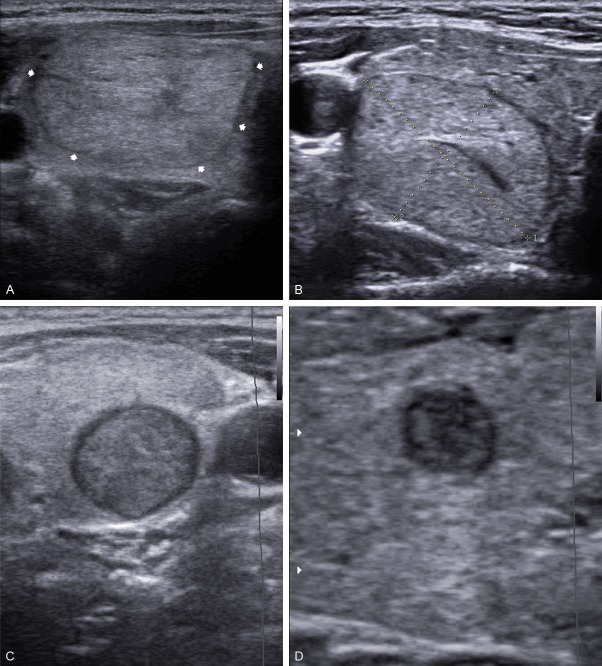
The main Sonographic criteria included echogenicity, margin, calcifications and vascularity of each thyroid nodule was evaluated based on previous publication. The echogenicity of each nodule was classified as hyperechogenicity, isoechogenicity, hypoechogenicity in comparison with the normal background thyroid tissue. Marked hypoechogenicity was defined as lower echogenicity than the cervical strap muscle (Figure 1A-D).
Figure 1.
Sonograms of thyroid show, A: Echogenic; B: Isoechoic; C: Hypoechoic; D: Marked Hypoechoic nodules.
The margin was characterized as well circumscribed (Hallo appearance) and not well-circumscribed (microlobulated/irregular) (Figure 2A, 2B).
Figure 2.
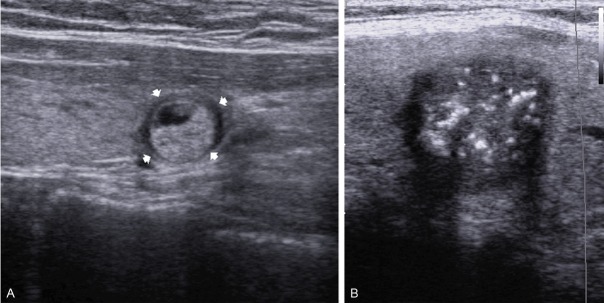
Sonograms of thyroid show, A: Well circumscribed echogenic nodule with Hallo margin; B: Hypoechoic thyroid nodule with irregular margin and cluster of microcalcification.
Calcifications were documented as microcalcifications, macrocalcifications, or without calcification. Microcalcifications were defined as tiny (1-2 mm in size), punctuate, and hyperechoic foci, either with or without posterior acoustic shadowing. Macrocalcifications were defined as larger than 2 mm (Figure 3A, 3B).
Figure 3.
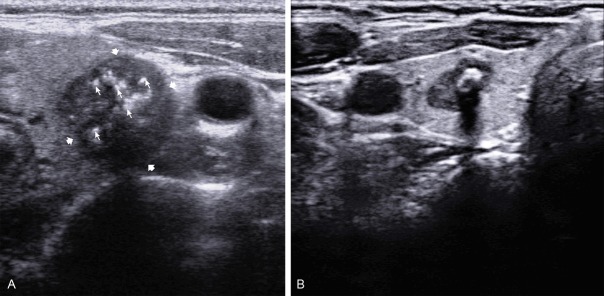
Sonograms of thyroid show, A: Hypoechoic thyroid nodule with marked microcalcification; B: Hypoechoic thyroid nodule with macrocalcification.
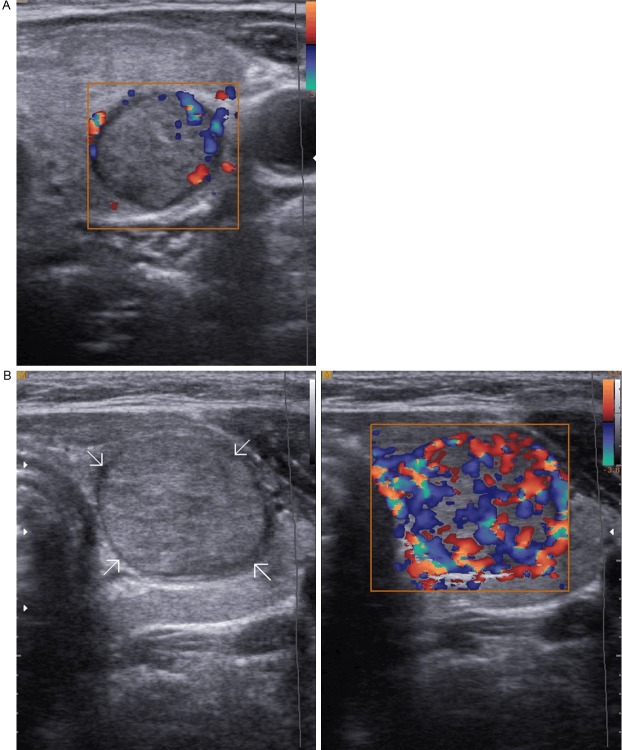
The vascularity of each nodule on color Doppler was classified as absent, peripheral, and central vascularity. When a nodule showed both peripheral and central vascularity, it was classified as central vascularity on color Doppler sonography (Figure 4A, 4B).
Figure 4.
Sonograms of thyroid show, A: Color Doppler ultrasonogram shows peripheral vascularity; B: Color Doppler ultrasonogram shows marked intranodular vascularity.
Each of the ultrasound appearance of the biopsied nodules was documented by one experienced radiologists in thyroid sonography.
The presence and absence of each of the above mentioned US feature as Hypoechogenicity, microcalcification, central vascularity and irregular margines without halo appearance were scored 2 and other US appearances were scored 0, respectively, then for each nodule, the total US score was documented.
FNAB was performed by an experienced radiologist using US guidance. Patients were placed supine with extended neck. In the first stage the lesion was localized and then the neck was prepared in a sterile environment. A 23-gauge needle attached to a 20-mL syringe and under observation, the needle tip was inserted perpendicularly to the neck until it be visible as a bright spot on the monitor, the needle was introduced to the thyroid nodule. This procedure was performed without local anesthetic. The needle aspiration was performed with to-and-fro movements in real time monitoring; suction was released before the needle was removed. A minimum of 4 needle passes were performed. After needle aspiration the specimen was placed, smeared, and fixed on glass slides and staining was performed with Papanicolaou and Giemsa stain.
Cytology was interpreted by one experienced thyroid cytopathologists and the results were reported as diagnostic or satisfactory and nondiagnostic or unsatisfactory. A specimen was considered diagnostic if there were at least 6 groups of benign follicular, each group composed of at least 10 cells. Cytopathology was reported as benign, indeterminate (follicular or Hurthle cell neoplasm), suspicious for malignancy, or malignant according to The Bethesda System for Reporting Thyroid Cytopathology. Benign cytology included colloid nodules, adenomatous nodules, lymphocytic thyroiditis, and other. The interpretation of “suspicious for malignancy” on cytology was defined when the specimen did not fulfill the criteria for a diagnosis of papillary carcinoma. Only those patients with definite Pathological results as benign or malignant were included in the study. Malignant results included papillary, follicular, medullary and undifferentiated thyroid carcinoma and others.
Cytologic results were considered malignant when the specimen showed abundant cells with cytologic features of cancer. The results of repeated biopsy were included in the data analysis.
When the cytological results from repeated FNAB were benign, those nodules were considered in the final diagnosis that repeated assessment by palpation or ultrasound in 12-months intervals showed no changes in size or morphology or repeated FNA in those patients with change in size or morphology of nodule confirmed the benign cytology.
Results
A total of 204 patients from 280 enrolled patients (175 women, 29 men) with 204 nodules (78 malignant and 126 benign nodules) had definite diagnosis according to FNAB results and were included.
Because surgery or repeated FNAB was not performed after initial FNAB, 76 of 280 nodules with nondiagnostic cytology were excluded from this study.
The mean age of our patients was 44.48 ± 13.04 years. The mean age of patients with benign nodules was 43.90 ± 12.63 years and mean age of patients with malignant nodules was 45.41 ± 13.8 years. There were no significant differences between malignant and benign thyroid nodule in respect of age (p = 0.1).
In our study the size of the nodules ranged from 5 mm to 45 mm (mean size of nodules was 17.3 mm ± 7.9). There was no significant difference between benign (6.92 ± 1.86 mm) and malignant nodules (5.82 ± 1.86 mm) (p = 0.07).
The cut off values of US score at maximum sensitivity and specificity for diagnosis of malignant nodule was 4 (two sonographic criteria) (Sensitivity: 67.9% and Specificity: 87%).
ROC analyses were performed to compare the diagnostic performance of different ultrasonographic feature and their predictive potential for discrimination of malignant nodule (Figure 5).
Figure 5.
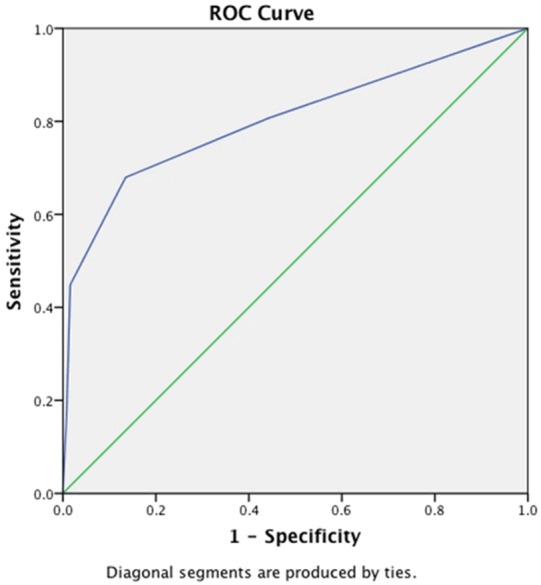
Receiver operating characteristic (ROC) curves was computed to compare the ability of different ultrasonographic feature to predict malignant thyroid nodule.
The sensitivity and specificity of US score for diagnosis of malignant nodules with one sonographic criteria (score = 2) was 80.8% and 55.6% respectively.
According to our study the sensitivity of hypoechogenicity, absence of halo, micro calcification and central vascularity in discrimination of malignant from benign thyroid nodule is 77.4% (CI 95% : 64.71-86.68%), 80.64% (CI 95% : 68.25%-89.18%), 46.16% (CI 95% : 32.67-58.28%) and 43.54% (CI 95% : 31.20-56.68%) respectively.
According to our study the sensitivity, negative predictive value and positive predictive value of hypoechogenicity, absence of halo, micro calcification and central vascularity in diagnosis of malignant thyroid nodule is as Table 1.
Table 1.
Diagnostic efficacy of the different US feature for discrimination malignant nodules
| Ultrasound feature | Sensitivity | Positive predictive value | Negative predictive value |
|---|---|---|---|
| Hypoechogenicity | 77.4% (CI 95% : 64.71-86.68%) | 90.76% | 73.23% |
| Absence of halo | 80.64% (CI 95% : 68.25-89.18%) | 91.11% | 69.87% |
| Microcalcification | 46.1% (CI 95% : 32.67-58.28%) | 84.98% | 87.35% |
| Intranodular vascularity | 43.54% (CI 95% : 31.20-56.68%) | 84.49% | 87.68% |
Discussion
Thyroid ultrasound (US) is the major diagnostic modality for detection of thyroid nodules and has a major role to exclude the presence of malignancy [7]. Regardless of size malignancy is found in approximately 5% of all thyroid nodules [2]. Because of the high prevalence of thyroid nodules especially in iodine deficient area, it is essential to determine an accurate and cost-effective guideline for pick up of suspicious thyroid nodules on the ultrasound features. Several ultrasound criteria as hypoechogenicity, absence of halo margin, microcalcifications, and an intranodular vascularity have been claimed as major predictors for the presence malignancy in thyroid nodules [8-10]. The above mentioned ultrasonographic features had relatively high specificity but low sensitivity in previous studies and there is no accurate single ultrasonographic feature for a recommendation of FNAB [11-14].
Leenhardt et al [11] revealed that hypoechogenicity has a moderate positive predictive value (50 to 63%) for malignancy in thyroid nodules and specificity (61 to 83%) for US examination. Li et al evaluated ultrasonographic feature of 104 patients with PTC and found that microcalcifications, intra nodular vascularity, and irregular borders were seen more common in malignant thyroid nodules [12]. Gonzalez-Gonzales evaluated the ultrasonographic feature of 341 thyroid nodules and revealed that microcalcification was the only reliable US feature that can significantly predict malignancy [13]. Moon et al [14] showed that intranodular vascularity is the most reliable Ultrasonographic feature of malignancy in thyroid nodules.
In our study, we found that although different sonographic criteria have been used for the prediction of malignancy in thyroid nodules but presence of two sonographic criteria have the best cut of value for sonographic prediction of suspicious thyroid nodules. Ozel et al [1] showed that cut of value of 2 and 3 have maximum sensitivity and specificity for nodules larger and smaller than one centimeter respectively. Kim et al [15] considered irregular or microlobulated margin, marked hypoechogenicity, microcalcifications and a shape (taller than wide) as sonographic criteria in non palpable thyroid nodules for discrimination of malignant from benign thyroid nodules they showed that. The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of prediction of malignancy based on one sonographic criteria were 93.8%, 66%, 56.1%, 95.9%, and 74.8%, respectively. They did not consider intranodular vascularity of thyroid nodule as a sonographic criterion for prediction of malignancy and had a low specificity in their study. Ahn et al [16] showed that FNAB should be performed in all of the hypo echoic nodules that are accompanied with at least with one of the following US criteria as taller than wide shape, irregular margins, microcalcifications, or intranodular vascularity. Yoon et al [17] showed that US-FNAB has false-negative rates of about 2% in prediction of malignancy.
According to Moon et al [18] study US has 77% diagnostic accuracy in depiction of malignant nodule when one of the US criteria of malignancy as a shape taller than wide, a spiculated margin, marked hypoechogenicity, microcalcification and macrocalcification were present. We believe that intranodular vascularity is an important criterion in prediction of malignancy and low diagnostic accuracy of their study may be due to lack of this finding in their inclusion criteria. In our study we evaluated the most accepted criteria for diagnosing malignant nodules in gray scale and Doppler ultrasonography irrespective to nodule size and found that at least two.
Ultrasound features have to be taken into consideration for FNAB.
Conclusion
Discrimination between benign and malignant thyroid nodules with US could be made with high sensitivity and specificity and presence of at least two US feature of malignancy may be the best predictor for FNAB recommendation. The practical use of this US score may help reduce unnecessary and expensive FNAB especially in iodine-deficient areas.
References
- 1.Ozel A, Erturk SM, Ercan A, Yılmaz B, Basak T, Cantisani V, Basak M, Karpat Z. The diagnostic efficiency of ultrasound in characterization for thyroid nodules: how many criteria are required topredict malignancy? Med Ultrason. 2012;14:24–8. [PubMed] [Google Scholar]
- 2.Gharib H, Papini E, Valcavi R, Baskin HJ, Crescenzi A, Dottorini ME, Duick DS, Guglielmi R, Hamilton CR, Zeiger MA, Zini M. American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2006;12:63–102. doi: 10.4158/EP.12.1.63. [DOI] [PubMed] [Google Scholar]
- 3.Hegedüs L. Clinical practice: the thyroid nodule. N Engl J Med. 2004;351:1764–1771. doi: 10.1056/NEJMcp031436. [DOI] [PubMed] [Google Scholar]
- 4.Hong YJ, Son EJ, Kim EK, Kwak JY, Hong SW, Chang HS. Positive predictive values of sonographic features of solid thyroid nodule. Clin Imaging. 2010 Mar-Apr;34:127–33. doi: 10.1016/j.clinimag.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Yoon JH, Kim EK, Hong SW, Kwak JY, Kim MJ. Sonographic features of the follicular variant of papillary thyroid carcinoma. J Ultrasound Med. 2008;27:1431–7. doi: 10.7863/jum.2008.27.10.1431. [DOI] [PubMed] [Google Scholar]
- 6.Popowicz B, Klencki M, Lewiński A, Słowińska-Klencka D. The usefulness of sonographic features in selection of thyroid nodules for biopsy in relation to the nodule’s size. Eur J Endocrinol. 2009;161:103–11. doi: 10.1530/EJE-09-0022. [DOI] [PubMed] [Google Scholar]
- 7.Lee YH, Kim DW, In HS, Park JS, Kim SH, Eom JW, Kim B, Lee EJ, Rho MH. Differentiation between benign and malignant solid thyroid nodules using an US classification system. Korean J Radiol. 2011;12:559–567. doi: 10.3348/kjr.2011.12.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cappelli C, Castellano M, Pirola I, Cumetti D, Agosti B, Gandossi E, Agabiti Rosei E. The predictive value of ultrasound findings in the management of thyroid nodules. QJM. 2007;100:29–35. doi: 10.1093/qjmed/hcl121. [DOI] [PubMed] [Google Scholar]
- 9.Salmaslioglu A, Erbil Y, Dural C, Issever H, Kapran Y, Ozarmagan S, Tezelman S. Predictive value of sonographic features in preoperative evaluation of malignant thyroid nodules in a multinodular goiter. World J Surg. 2008;32:1948–1954. doi: 10.1007/s00268-008-9600-2. [DOI] [PubMed] [Google Scholar]
- 10.Horvath E, Majlis S, Rossi R, Franco C, Niedmann JP, Castro A, Dominguez M. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. 2009;94:1748–1751. doi: 10.1210/jc.2008-1724. [DOI] [PubMed] [Google Scholar]
- 11.Leenhardt L, Tramalloni J, Aurengo H, Delbot T, Guillausseau C, Aurengo A. Échographie des nodules thyroidiens: l’echographiste face aux exigencies du clinician. Presse Med. 1994 Oct 8;23:1389–92. [PubMed] [Google Scholar]
- 12.Li QS, Chen SH, Xiong HH, Xu XH, Li ZZ, Guo GQ. Papillary thyroid carcinoma on sonography. Clin Imaging. 2010;34:121–6. doi: 10.1016/j.clinimag.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 13.González-González A, Mate Valdezate A, Parra Arroyo A, Tenías Burillo JM. Diagnostic efficiency of sonographic findings of thyroid nodules in the detection of malignancy. Endocrinol Nutr. 2010;57:240–4. doi: 10.1016/j.endonu.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Moon HJ, Kwak JY, Kim MJ, Son EJ, Kim EK. Can vascularity at power Doppler US help predict thyroid malignancy? Radiology. 2010;255:260–9. doi: 10.1148/radiol.09091284. [DOI] [PubMed] [Google Scholar]
- 15.Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, Yoo HS. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178:687–91. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- 16.Ahn SS, Kim EK, Kang DR, Lim SK, Kwak JY, Kim MJ. Biopsy of thyroid nodules: comparison of three sets of guidelines. AJR Am J Roentgenol. 2010;194:31–37. doi: 10.2214/AJR.09.2822. [DOI] [PubMed] [Google Scholar]
- 17.Yoon JH, Kwak JY, Moon HJ, Kim MJ, Kim EK. The diagnostic accuracy of ultrasound-guided fine-needle aspiration biopsy and the sonographic differences between benign and malignant thyroid nodules 3 cm or larger. Thyroid. 2011;21:993–1000. doi: 10.1089/thy.2010.0458. [DOI] [PubMed] [Google Scholar]
- 18.Moon HJ, Kwak JY, Kim EK, Kim MJ, Park CS, Chung WY, Son EJ. Benign and malignant thyroid nodules: US differentiation-multicenter retrospective study. Radiology. 2008;247:762–770. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]