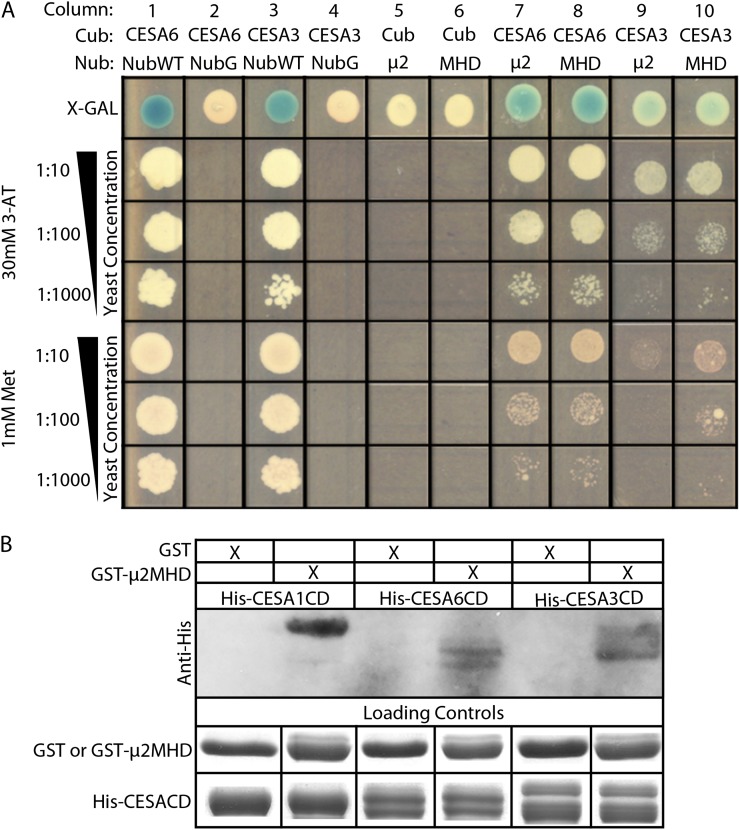
Figure 1.
μ2 interacts with primary CESAs through the μ-homology domain. A, SU-Y2H analysis shows a positive interaction between μ2 and both CESA3 and CESA6. Interactions were selected on Leu-, Trp-, and His-dropout medium with 1.0 mm Met or 30 mm 3-ammonium-triazole (3-AT). 5-Bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was added for the detection of β-galactosidase activity. Yeast strains were cotransformed with a Cub vector and a Nub vector. Cub vectors consisted of CESA6-Cub-PLV, CESA3-Cub-PLV, or Cub-PLV alone as a negative control. Nub vectors consisted of μ2-NubG or μ2MHD-NubG as experimental constructs and NubWT alone and NubG alone as positive and negative controls, respectively. B, μ2MHD interacts with the central domain of CESA1, CESA3, and CESA6 in vitro. His-CESA1CD, His-CESA3CD, and His-CESA6CD all coprecipitated with GST-μ2MHD. GST alone did not pull down His-CESA1CD, His-CESA3CD, or His-CESA6CD.