Table 2. Ortho-Iodination of Phenylacetic Amidesa ,b, c, d, e.
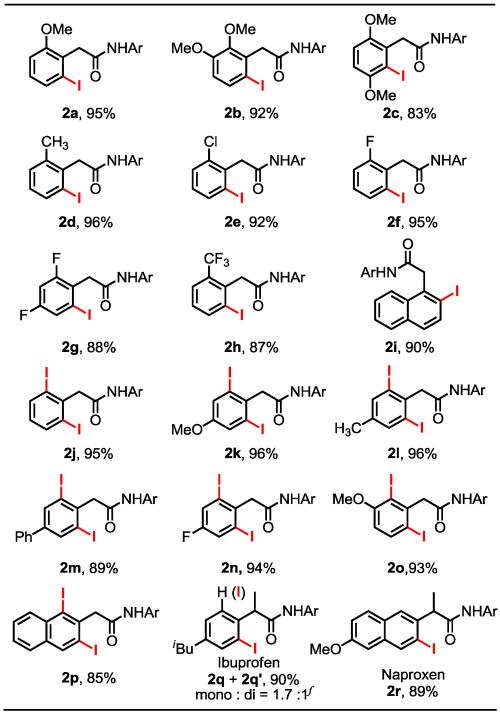
|
Ar = (4-CF3)C6F4.
Reaction conditions for mono-iodination: 0.30 mmol of phenylacetic amide, 2 mol % Pd(OAc)2, 0.75 mmol I2, 0.36 mmol CsOAc, 0.30 mmol NaHCO3, 150 mg 4 Å molecular sieves and 5.0 mL of t-AmylOH/DMF (1:1) in a sealed tube, 65 °C, 20 h.
For products 2g, 2h and 2r, 5 mol % Pd(OAc)2 was used.
Reaction conditions for di-iodination: 0.10 mmol of phenylacetic amide, 5 mol % Pd(OAc)2, 0.50 mmol I2, 0.24 mmol CsOAc, 0.20 mmol NaHCO3, 50 mg 4 ° molecular sieves and 2.0 mL of t-AmylOH/DMF (1:1) in a sealed tube, 65 °C, 20 h.
Yields of the isolated product.
Ratio was determined by the 1H NMR spectroscopy.
