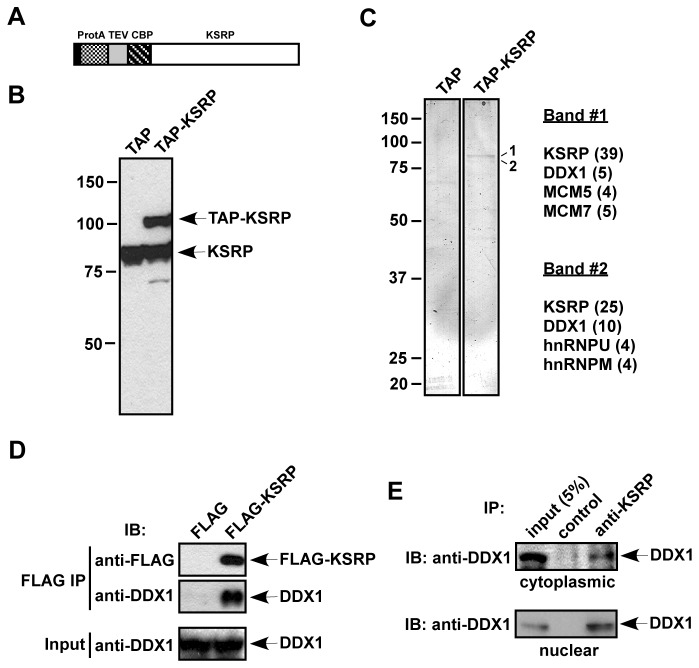
Figure 1. Purification of KSRP-associated proteins.
(A) A schematic diagram of TAP-KSRP. Sequences encoding the TAP tag containing protein A (ProtA), a TEV protease cleavage site, and a calmodulin binding peptide (CBP) were fused to the N-terminus of KSRP. (B) Extracts from HT1080 stable cells expressing the TAP tag or TAP-KSRP were analyzed by anti-KSRP immunoblotting. (C) Extracts containing TAP and TAP-KSRP were treated with RNase A and subjected to TAP purification. The purified fractions were analyzed by silver staining. Two bands detected only in the TAP-KSRP fraction are labeled (1 and 2). Proteins identified from bands 1 and 2 by mass spectrometry are indicated. Numbers of observed peptides are denoted in parentheses. (D) FLAG or FLAG-KSRP was expressed in HeLa-TO cells. RNase A-treated extracts were subjected to anti-FLAG immunoprecipitation. The precipitates were analyzed by anti-FLAG or anti-DDX1 immunoblotting. 5% of input used for immunoprecipitation was also analyzed by anti-DDX1. (E) RNAse A-treated cytoplasmic and nuclear extracts were immunoprecipitated with an anti-KSRP monoclonal antibody or a control IgG. The precipitates and 5% of input were analyzed by anti-DDX1 immunoblotting.