Abstract
The αvβ6 integrin is up-regulated in cancer and wound healing but it is not generally expressed in healthy adult tissue. There is increasing evidence that it has a role in cancer progression and will be a useful target for antibody-directed cancer therapies. We report a novel recombinant diabody antibody fragment that targets specifically αvβ6 and blocks its function. The diabody was engineered with a C-terminal hexahistidine tag (His tag), expressed in Pichia pastoris and purified by IMAC. Surface plasmon resonance (SPR) analysis of the purified diabody showed affinity in the nanomolar range. Pre-treatment of αvβ6-expressing cells with the diabody resulted in a reduction of cell migration and adhesion to LAP, demonstrating biological function-blocking activity. After radio-labeling, using the His-tag for site-specific attachment of 99mTc, the diabody retained affinity and targeted specifically to αvβ6-expressing tumors in mice bearing isogenic αvβ6 +/− xenografts. Furthermore, the diabody was specifically internalized into αvβ6-expressing cells, indicating warhead targeting potential. Our results indicate that the new αvβ6 diabody has a range of potential applications in imaging, function blocking or targeted delivery/internalization of therapeutic agents.
Introduction
The αvβ6 integrin is an epithelial restricted trans-membrane protein that has emerged as a promising target for antibody-directed therapies. It is up-regulated in many tumor types including pancreatic ductal adenocarcinoma, head and neck squamous cell carcinoma, ovarian cancer, colon cancer, cholangiocarcinoma and cervical cancer [1], [2], [3], [4], [5]. During embryogenesis and wound healing αvβ6 promotes binding to extracellular matrix proteins (fibronectin, vitronectin and tenascin) facilitating cell migration [6] and activates TGFβ1 via binding to the latency associated peptide (LAP) of the TGFβ complex [7]. In cancer, αvβ6 has been shown to modulate invasion, inhibit apoptosis and regulate expression of matrix metalloproteases (MMPs) [8]. Importantly for a cancer target, αvβ6 is only found at very low levels in normal tissue; its expression has been reported to regulate wound healing [6] and activation of TGFβ1 in response to injury and inflammation in the lungs [9].
The various roles of αvβ6 in cancer have not yet been fully elucidated, although it has been shown to be a contributing factor in tumor progression [4], [10] and has been associated with enhanced tumorigenic properties in colon carcinoma facilitating liver metastasis [4], [11], and reducing survival times in gastric carcinoma [10]. Expression of αvβ6 has been reported during epithelial-mesenchymal transition (EMT) and it is thought to have a role in sustaining the EMT process [12], [13]. Interestingly, high levels of αvβ6 are found in the context of K-Ras dependency in lung and pancreatic cancer cell lines [14]. Depletion of the ITGB6 gene had a clear growth inhibitory effect on these cells [15], indicating that αvβ6 may be a tractable target in K-Ras mutant cancers. Targeting this integrin has shown tumor growth inhibition in vivo due to blockade of αvβ6-dependent activation of the TGFβ pathway [16].
Antibodies reactive specifically with αvβ6 could have diagnostic and therapeutic utility, particularly if they have function blocking activity. Towards this, we previously engineered murine and humanized single chain Fv antibody fragments (scFvs) reactive with αvβ6 [17]. The unique αvβ6 specificity was gained by an insertion into the CDR3 loop of the variable heavy-chain (VH) domain of an existing scFv scaffold. Here we describe the development of an anti-αvβ6 scFv into a stable in vivo targeting agent in diabody format. Diabodies are non-covalently associated bivalent molecules, created from scFvs by shortening the polypeptide linker between the VH and VL domains [18]. Their bivalent nature is advantageous for targeting [19], [20], [21] and they provide a flexible platform for development of targeted therapeutics, particularly since their pharmacokinetics are readily modified by attachment of polyethylene glycol [22]. We show that the anti-αvβ6 diabody blocks αvβ6-mediated biological functions. Moreover, the 99mTc-labeled diabody targeted specifically to αvβ6+ve tumors in vivo within 2 hours of administration.
Materials and Methods
Cell lines
A375Pβ6 is a αvβ6-positive human cell line, generated through retroviral transduction of the melanoma cell line A375P with human β6 cDNA and a puromycin-resistance gene as described previously [23]. The control cell line, A375Ppuro was transduced with the puromycin-resistance gene alone [23]. Both cell lines express several other RGD-binding integrins at equivalent levels, namely α5β1, αvβ3, αvβ5, αvβ8 [17]. Capan-1 (αvβ6-positive human pancreatic cell line) was obtained from ATCC (HTB-79). All cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) (PAA Laboratories, UK) supplemented with 2 mM L-glutamine (PAA Laboratories, UK) and 10% foetal calf serum (Labtech International, Ringmer, UK).
Production of B6.3 and shMFE23 diabody proteins
The diabody was generated from the B6.3 scFv vH and vL domains [17] by synthesizing the scFv gene with a G4S linker. The synthesized gene was obtained from Genescript (Piscataway, NJ, USA) and cloned into the pPICZαBHis vector (Invitrogen) as described previously [17]. The resulting plasmid was linearized with PmeI, transformed into electrocompetent P. pastoris X33 cells (Invitrogen) and transformants grown on YPDS and Zeocin (100 μg/ml; Invitrogen) plates. Positive clones were selected and screened for methanol-induced protein expression according to the manufacturer's recommendations. Clones with the highest B6.3 diabody expression were used for protein production by fermentation with initial purification using expanded-bed adsorption IMAC as previously described [24], [25]. The B6.3 diabody was harvested 4 h post induction of protein expression. Final purification was performed by size-exclusion chromatography on a Superdex 75 (GE Healthcare) column (500-ml bed volume) equilibrated with phosphate-buffered saline (PBS), pH 7.4. For 99mTc labeling experiments the diabody was further concentrated to 7.3 mg/ml by application to a 1ml Ni2+-charged HiTrap IMAC SP FF column (GE Healthcare) according to the manufacturer's instructions. Purified protein was analyzed by SDS-PAGE using Tris-glycine gels (16%; Invitrogen) and stained with Coomassie brilliant blue R250 (Sigma). The shMFE23 diabody was produced following the same protocol as for B6.3 diabody production, using the shMFE23 scFv [26] vH and vL domains as template. The purified protein gave a single peak by size exclusion chromatography (data not shown) indistinguishable from that obtained with the B6.3 diabody.
Affinity of αvβ6 binding to B6.3 diabody by Surface Plasmon Resonance
Affinity of purified B6.3 diabody for αvβ6 was measured by surface plasmon resonance (SPR) using a Biacore T100. The diabody was immobilized on a Research Grade CM5 chip using an amine coupling kit (BIAcore, GE Healthcare). Recombinant αvβ6 protein (R&D Systems) was flown over the immobilized B6.3 diabody in HBS-P buffer (10 mM HEPES, 150 mM NaCl, 0.05% v/v Surfactant P20, pH 7.4, with addition of 2 mM Ca2+ and 2 mM Mg2+ ions) at 30 μL/min at 25°C. Association and dissociation phases occurred over 300 s. Kinetics of binding was calculated from data at 400 nM, 200 nM, 100 nM, 50 nM, 25 nM, 12.5 nM, 6.25 nM and 3.125 nM using the BIAevaluation program. The surface was regenerated with 10 mM Glycine-HCl, pH 2.5. The affinity constant (KD) was obtained by simultaneously fitting the association and dissociation phases of the sensogram from the analyte concentration series using the 1∶1 Langmuir model (BIAevaluate software).
Flow cytometric analysis of B6.3 diabody binding to αvβ6-expressing cells
A375Pβ6 and A375Ppuro cells were trypsinized, re-suspended in DMEM supplemented with 0.1% (v/v) BSA and 0.1% (w/v) sodium azide (DMEM0.1/0.1) to approximately 5×106 cells/ml and incubated with various concentrations of B6.3 diabody. Bound diabody was detected with mouse Tetra-His antibody (1 μg/ 100 μl, Qiagen) and R-PE-conjugated goat anti-mouse IgG (BD Pharmingen, 1 μg/100μl). Detection antibodies were incubated in DMEM0.1/0.1 for 45 min at 4°C; all incubations were followed by washing with DMEM0.1/0.1. Cells were fixed with IntraStain kit (DakoCytomation, Glostrup, Denmark) and analyzed by flow cytometry using a CyAn ADP High-Performance Flow Cytometer (Becton Dickinson). For binding inhibition studies, A375Pβ6 cells were incubated with mouse anti-αvβ6 (10D5, Chemicon International) at various concentrations for 15vmin followed by incubation with 100 ng (18.04 nM) of diabody for 30 min. After washing, bound diabody was detected with rabbit anti-hexahistidine IgG (GenScript) at 1 μg/100 μl, followed by R-PE-conjugated goat anti-rabbit IgG (1 μg/100 µl, Invitrogen). All incubation and washing steps were in DMEM0.1/0.1 at 4°C. Cells were fixed and analysed as described above.
99mTc labeling of B6.3 diabody
Sodium [99mTc] pertechnetate was obtained from a 99Mo/99mTc generator (GE Healthcare, Amersham UK) and converted to [99mTc(CO)3(H2O)3]+using an IsoLinkTM kit (generously provided by Covidien, Petten, The Netherlands) according to the manufacturer's instructions. B6.3 diabody was labeled at the C-terminal hexahistidine tag with 99mTc by incubating with 750MBq of [99mTc(CO)3(H2O)3]+ in a total volume of 574 µl at 37°C for 2 h. The labeled protein was separated from the non-incorporated radionuclide by desalting (NAP-10 column, GE Healthcare). Integrity of the radio-labeled protein as a dimer was verified by size-exclusion HPLC on a Biosep-SEC-S 2000 column eluted with 0.1 M phosphate buffer pH 7 at a flow rate of 0.5 ml/min.
Cell Saturation Binding Assay
The immunoreactivity and affinity of 99mTc-labeled B6.3 diabody to αvβ6 was analyzed by a saturation-binding assay using A375Pβ6 cells. Six duplicate test samples containing increasing amounts of 99mTc-labeled B6.3 diabody and approximately 6.5×105 A375Pβ6 cells per experiment were incubated in a total volume of 1ml of DMEM with 0.1% (v/v) BSA (DMEM0.1) at 4°C for 3 h. Supernatant was removed by centrifugation and cells were washed once with DMEM0.1. An identical series of tubes were prepared in which non-specific binding was determined by addition of 25 µg unlabeled diabody to each tube. Non-specific binding was subtracted from total binding to obtain specific binding. Affinity constant (KD) and maximal number of αvβ6 binding sites (Bmax) were determined by non-linear regression analysis using Graphpad prism software.
Immunofluorescence microscopy of internalization of B6.3 diabody into αvβ6-expressing cells
A375Pβ6 cells were seeded on to glass cover slips at 2×105 cells/well and incubated for 48 h at 37°C. Cells were then washed with DMEM0.1, incubated with 5 µg/ml of B6.3 diabody in 1%BSA/DMEM (DMEM1) for 1 h at 4°C and subsequently washed and incubated in 10% (v/v) FBS/DMEM at 37°C for various time points. After incubation, cells were washed twice with Tris-Cl, pH 7.5, containing 2 mM Ca2+ and 1 mM Mg2+ (Tris/M), followed by fixation in 4% paraformaldehyde/Tris/M for 20 min on ice. After washing with PBS, cells were incubated with 10 mM ammonium chloride/PBS for 10 min at room temperature and permeabilized with ice-cold methanol. Finally, cells were blocked with 1% (w/v) BSA/PBS for 30 min at room temperature and stained with 1 μg/ml of rabbit anti-human IgG (Jackson Immuno Research, Suffolk, UK) in 1% (w/v) BSA/PBS followed by Alexa Fluor 546®-labeled goat anti-rabbit IgG (1∶500) (Invitrogen), containing Hoechst trihydrochloride (1∶5000) (Invitrogen) in 1% (w/v) BSA/PBS, each for 1 h at 4°C. Cover slips were mounted on slides using ProLong Gold antifade (Invitrogen) and examined using Perkin Elmer Spinning Disc Confocal microscope and VolocityTM Visualisation Software.
Adhesion assays
Ninety-six-well plates were coated with 100 µl of fibronectin (R&D Systems) at 25 µg/ml or LAP (R&D Systems) at 0.5 µg/ml for 1 h at 37°C. After coating, plates were washed with PBS and blocked with 1%BSA/PBS at 37°C for 1 h. For blocking experiments, cells were treated with 50 µg/ml B6.3 diabody for 1 h at 4°C in DMEM0.1 and seeded at 5×104 cells/well. After incubation at 37°C for 1 h, plates were extensively washed with PBS to remove non-attached cells and 100 µl of a dilution 1∶10 of Prestoblue© (Invitrogen) was added to each well. Fluorescence signal was measured after incubation at 37°C for 4 h using a Multimode Varioskan plate reader (Thermo Scientific). Results were expressed as percentage of attachment relative to untreated cells with statistical significance analyzed by Student's unpaired t-test.
Migration assays
Cell migration was analysed using Transwell assays (Corning, NY, USA) with polycarbonate filters (8 µm pore size). Membrane undersurface was coated with fibronectin (R&D Systems) at 25 µg/ml or LAP (R&D Systems) at 0.5 µg/ml for 1h at 37°C and blocked with DMEM0.1 for 1 h at 37°C. Cells were treated as described for adhesion assays and seeded in the upper chamber at 1×105 cells/chamber in 100 µl. The lower chamber was filled with 600 µl DMEM0.1. Plates were then incubated for 20 h at 37°C and cells in the upper chamber were carefully removed using a cotton swap. Migrated cells were fixed with 4% paraformaldehyde, stained with Hoechst trihydrochloride (1∶5000) (Invitrogen) for 10 min and counted using a Zeiss AxioImager A1 fluorescence microscope with AxioVision software. Results were expressed as percentage of migrated compared to untreated cells with statistical significance analyzed by Student's unpaired t-test.
Immunocytofluorescence for Smad2/3 localization
Capan-1 cells were seeded in cover slips in 10% (v/v) FBS/DMEM and allowed to grow to 70% confluency. Cells were then washed twice in PBS, starved for 24 h in serum-free DMEM and treated with 50 µg/ml B6.3 diabody for 1 h at 4°C in DMEM0.1. After incubation with the diabody cells were washed twice with PBS and treated with DMEM0.1, latent TGFβ1 (Cell Signaling Technology) at 50 ng/ml or TGFβ1 (R&D Systems) at 10 ng/ml for 30 min at 37°C. After extensive washing with PBS, 4% formaldehyde was used to fix the cells (15 min at 4°C). Cells were then permeabilized with 0.3% Triton-X 100 (Sigma) in PBS and cover slips were blocked for 1 h with 5% goat serum before overnight incubation with rabbit anti-Smad2/3 antibody (Cell Signaling Technology) at 4°C. The following day, primary antibody was detected with Alexa Fluor 488®-labeled goat anti-rabbit IgG (Invitrogen) containing Hoechst trihydrochloride (Invitrogen). Cover slips were mounted on slides using ProLong Gold antifade (Invitrogen) and examined using a Zeiss AxioImager A1 fluorescence microscope with AxioVision software.
In vivo studies
All experiments were conducted with previous approval from the UK Home Office, under PPL 70/6677. Female SCID mice were injected subcutaneously (s.c.) with 4×106 A375Pβ6 cells in one flank, and 4×106 A375Ppuro cells in the contralateral flank, in 150 µl serum-free DMEM. Once tumors reached a diameter of around 5 mm, approximately 11 µg (30MBq) per mouse of 99mTc-labeled B6.3 diabody in 200 µl PBS was injected intravenously (i.v.). Mice were anaesthetised with isoflurane and imaged 2 h, 5 h and 24 h after injection using a Nano-SPECT/CT scanner (Bioscan, Washington, DC, USA). SPECT images were analysed using in vivo Scope software (Bioscan). Mice were sacrificed 24 h after injection of diabody; tissues were excised and radioactivity measured on a gamma counter (LKB Compugamma, Victoria, Australia) alongside standards prepared from the injectate. Uptake of radioactivity in individual tissues was expressed as a percentage of the injected radioactive dose per gram (%ID/g).
Results
Expression and characterisation of B6.3 diabody
B6.3 diabody was generated as soluble protein by fermentation in P. pastoris giving a yield of 175 mg/L. The diabody was purified from the bioreactor broth using expanded bed IMAC, exploiting the engineered hexahistidine tag, and concentrated to 2.27 mg/ml. There was no evidence of aggregation when the product was tested by size-exclusion chromatography; diabody eluted as a single peak of >44 kDa, consistent with its calculated MW of 55,322Da (Fig. 1A). The protein was essentially pure as shown by SDS-PAGE and was revealed as a monomer under denaturating conditions (Fig. 1A), consistent with diabody formation by non-covalent association. We next analysed the binding affinity of the purified B6.3 diabody to αvβ6. SPR showed that αvβ6 bound to B6.3 diabody in a concentration-dependent manner (Fig. 1B) and subsequently remained associated. Fitting of the data to a Langmuir 1∶1 model gave an affinity constant (KD) value of 2.78−10−9M and kinetic rate constants of ka = 8.1×103±7.3 s−1M−1 and kd = 2.3×10−5±1.4×10−7s−1.
Figure 1. Production of B6.3 diabody and analysis of its specific interaction with αvβ6.
A) Size-exclusion chromatographic profile (Superdex 75, 125 ml) of B6.3 diabody after fermentation, expanded-bed adsorption IMAC, Superdex 75 (500 ml), 1 ml Ni2+-charged Hi-Trap IMAC, freezing and de-frosting. B6.3 diabody eluted from the column as a dimer that separated in monomeric form under reducing conditions by SDS-PAGE, consistent with non-covalent association of monomers in a diabody structure. B) Sensogram of real-time binding and dissociation of B6.3 diabody to αvβ6. B6.3 diabody was immobilized on a BIAcore CM5 sensor chip and αvβ6 protein was flown across at 400, 200, 100, 50, 25, 12.5, 6.25 and 3.125 nM. The affinity constant (KD) for the interaction was 2.8×10−9 M, with on-rate of 8,107±7.3 M−1s−1 and off-rate of 2.3×10−5±1.4×10−7 s−1. C) Flow cytometry analysis of B6.3 diabody binding to αvβ6-expressing A375Pβ6 cells in a concentration-dependent manner (a) but not to A375Ppuro cells (b), which do not express this integrin. Cells were incubated with B6.3 diabody, at the indicated concentrations and binding was detected with mouse anti-tetra-histidine IgG followed by R-PE-labeled goat anti-mouse IgG. B6.3 diabody was not added to omission control (shown in solid grey). D) Inhibition of B6.3 diabody binding after incubation with the anti-αvβ6 antibody 10D5 shown by flow cytometry. Cells were incubated with 100 ng B6.3 diabody with or without prior incubation with 10D5 at the indicated concentrations. Binding of B6.3 diabody was detected with rabbit anti-hexahistidine IgG followed by R-PE-labeled goat anti-rabbit IgG. In the omission control experiment (shown in solid grey) cells were not incubated with B6.3 diabody and 10D5.
Interactions with αvβ6-expressing cells
Specificity of purified B6.3 diabody for αvβ6 on tumor cells was assessed by flow cytometry using the αvβ6-expressing cell line, A375Pβ6, and corresponding αvβ6-negative A375Ppuro cells. The results showed that B6.3 diabody bound to the αvβ6-expressing cells in a concentration-dependent manner (Fig. 1C) but did not bind to the αvβ6-negative cells when tested at the two highest concentrations (Fig. 1C). The shift in fluorescence intensity observed for binding to A375Pβ6 cells were similar at 22.6, 45.1 and 90.2 nM, indicating that antigen saturation was reached at these concentrations. To further verify the specificity of the B6.3 diabody to αvβ6, cells were pre-incubated with an anti-αvβ6 antibody, 10D5. This resulted in inhibition of B6.3 diabody binding when 10D5 was used at 10 and 100 nM (Fig. 1D).
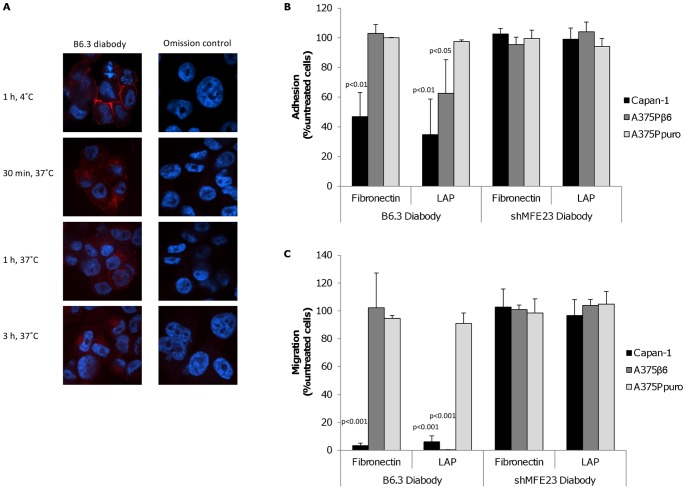
Next we tested whether the diabody would internalize specifically into the αvβ6-expressing cells, as previously reported for other ligand-mimic antibodies targeting this integrin [27]. Cells were treated with B6.3 diabody at 4°C for 1 h to allow binding to the outer cell membrane. Then, temperature was increased to 37°C for different length of time to allow internalization and the resulting cellular distribution of the diabody was revealed after fixation and permeabilization by fluorescence staining. Results of these experiments showed that, upon binding at 4°C, B6.3 diabody was localized at the cell surface (Fig. 2A). When the temperature was raised to 37°C surface staining disappeared and the diabody was found inside the cells after 30 min, 1 h and 3 h of incubation (Fig. 2A).
Figure 2. Treatment of αvβ6-expressing cells with B6.3 diabody resulted in diabody internalization and blockade of integrin functions.
A) Localization of B6.3 diabody in A375Pβ6 cells by confocal microscopy. B6.3 diabody detection showed membrane pattern of staining at 4°C and internalized when cells were incubated at 37°C for 30 min, 1 h and 3 h. B6.3 diabody was detected using rabbit anti-human IgG followed by Alexa Fluor 546®-labeled goat anti-rabbit IgG (red). Cells were also counterstained with Hoechst 33245 (blue). B) Treatment of αvβ6-expressing cells blocked adhesion to LAP-coated plates (A375Pβ6 and Capan-1 cells) and/or fibronectin-coated plates (Capan-1 cells). Cells were incubated with B6.3 or shMFE23 diabody at 4°C for 1 h and allowed to attach to coated plates for 1 h at 37°C. Treatment with the anti-CEA shMFE23 diabody had no effect on the cell lines used. C) B6.3 diabody treatment inhibited migration towards LAP and fibronectin. As observed in adhesion assays, the diabody inhibited migration of A375Pβ6 cells to LAP and migration of Capan-1 cells to fibronectin and LAP, while targeting CEA had no effect on the cells tested.
B6.3 diabody-mediated blockade of αvβ6 biological functions
The αvβ6 integrin is known to have a role in the promotion of cell migration based on interaction with components of the extracellular matrix [6], [11], [16]. Since the B6.3 diabody contained an RGD motif and internalized as a ligand-mimic antibody, we investigated whether the diabody would exhibit biological effects associated with integrin blockade. First we tested the ability of the diabody to inhibit adhesion and migration of αvβ6-positive or negative cells to the αvβ6 ligands, LAP and fibronectin. In addition to the stably-transfected A375Pβ6 cells, we included the naturally αvβ6-expressing pancreatic cancer cell line Capan-1. Treatment with B6.3 diabody at 50 µg/ml resulted in a reduction in adhesion of A375Pβ6 and Capan-1 cells to LAP-coated plates (Fig. 2B). In addition, we observed a decrease in the number of Capan-1 cells attached to fibronectin-coated plates. The shMFE23 diabody targeting the carcinoembryonic antigen (CEA) was used as control; pre-treatment with this diabody had no effect on the CEA-positive Capan-1 cells or the melanoma cell lines, negative for CEA [17]. The results from Transwell migration assays were more marked as the B6.3 diabody induced an almost complete inhibition of migration towards LAP in both A375Pβ6 and Capan-1 cells (Fig. 2C). In addition, the migration of Capan-1 cells to fibronectin was almost completely inhibited by the diabody, indicating that αvβ6 is the major fibronectin-binding integrin on Capan-1. In contrast, B6.3 diabody did not block A375Pβ6 cells adhering to or migrating on fibronectin (Fig 2C), consistent with our previous data that showed both A375Pβ6 and A375Ppuro express two other fibronectin-binding integrins αvβ3 and α5β1 [17]. No effect on adhesion or migration was observed in the αvβ6-negative cell line A375Ppuro or after addition of an irrelevant diabody, indicating that the B6.3-dependent inhibition was αvβ6-specific.
The αvβ6 integrin is known to activate TGFβ1 upon interaction with its latent form (LAP-TGFβ1 complex), resulting in TGFβ-induced smad2/3 phosphorylation and subsequent translocation of smad2/3 to the nucleus [7]. We hypothesized that binding of B6.3 diabody to αvβ6 would inhibit its interaction with latent TGFβ1 and downstream Smad2/3 translocation. We showed that smad2/3 localized in the cytoplasm in serum-starved Capan-1 cells (Fig. 3(a)). Incubation with B6.3 diabody had no effect on smad2/3 localization (Fig. 3(c)), while treatment with latent TGFβ1 resulted in nuclear translocation of Smad2/3 (Fig. 3(b)). We next tested the localization of smad2/3 after incubation with the B6.3 diabody. Our data showed that B6.3 diabody inhibited Smad2/3 nuclear translocation mediated by latent TGFβ1 (Fig. 3(d)), indicating that the diabody blocked smad2/3 activation. Active TGFβ1 showed Smad2/3 translocation in B6.3-treated cells (Fig. 3(f)), since the active form does not require prior integrin interactions.
Figure 3. B6.3 diabody inhibited LAP-mediated Smad2/3 translocation to the nucleus in Capan-1 cells.
Cells were incubated at 4°C in the presence of B6.3 diabody and then treated with LAP or TGFβ1 (30 min, 37°C); Smad2/3 localization was assessed by confocal microscopy (40X) using rabbit anti-Smad2/3 followed by Alexa Fluor 488®-labeled goat anti-rabbit IgG (green); smad2/3 was found in the cytoplasm of starved Capan-1 cells (a) and after treatment with B6.3 diabody (c). Smad2/3 was present in the nuclei in response to treatment with latent TGFβ1 (b), a translocation that was inhibited by pre-treatment B6.3 diabody (d). TGFβ1 was used as a positive control (e.f).
Labeling efficiency of B6.3 diabody
In order to determine the efficacy of the diabody for αvβ6 targeting in vivo, B6.3 diabody was labeled with 99mTc. This radionuclide was chosen as it has been described previously to be appropriate for use with internalizing antibody fragments [28] and the chemistry for conjugation to the hexahistidine tag is commercially available using the IsoLinkTm kit. After labelling, the resulting 99mTc-labeled diabody had a specific activity of 2.7MBq/µg and remained a dimer when tested by size-exclusion chromatography (data not shown). Strength and specificity of interaction of 99mTc-labeled diabody with purified αvβ6 protein was evaluated using a saturation binding experiment which revealed a concentration-dependent increase of 99mTc-B6.3 diabody (Fig. 4A). The specificity of interaction was tested by inhibition of binding by unlabeled diabody. The non-specific binding obtained from the inhibition experiments was subtracted from the total radioactivity to obtain specific binding. When tested on cells, the affinity constant derived from non-linear regression analysis of 99mTc-labeled B6.3 diabody was found to be in the nanomolar range, at 4.88±0.32×10−8 M. The maximal number of αvβ6 binding sites (Bmax) was derived to be 2.3±0.039×105 per cell. The Scatchard analysis, which showed a linear correlation (Fig. 4B), was in agreement with a single affinity binding site for the interaction.
Figure 4. Labeling with 99mTc did not affect B6.3 diabody binding to αvβ6.
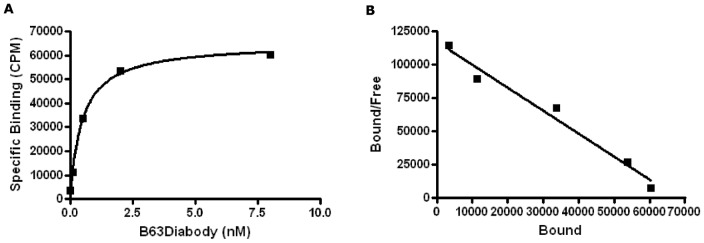
A) Saturation Binding experiment showed concentration-dependent binding of 99mTc-labeled diabody to A375Pβ6 cells. Non-specific binding, including 25 µg of unlabeled diabody was subtracted from each data point. KD obtained was 4.88±0.32×10−8 M and BMax was 2.3±0.039×105 receptors/cell (325±5.53 pM/8.5×105 cells). B) Scatchard presentation of the data. Each experiment was carried out in duplicate.
Targeting of B6.3 diabody to αvβ6-expressing tumors in vivo
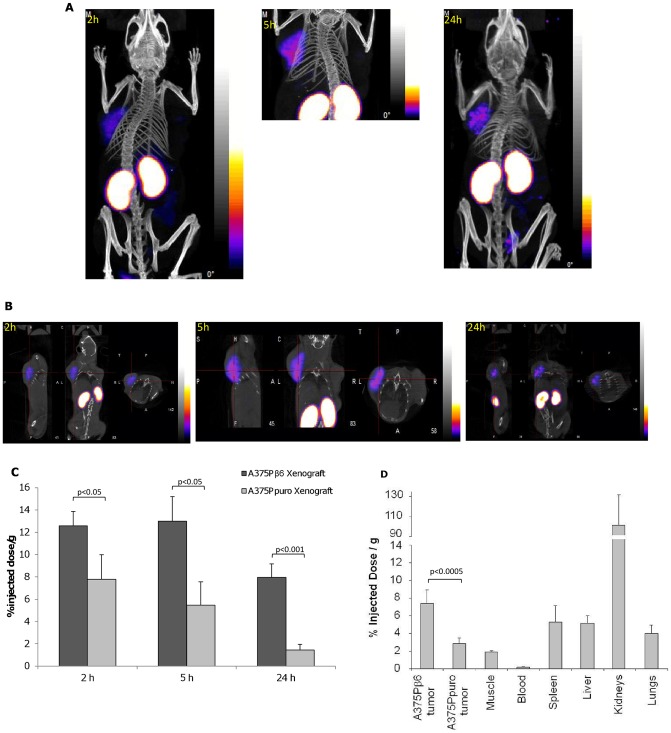
To determine the specificity of the B6.3 diabody in vivo, 99mTc-labeled diabody was administered to SCID mice bearing flanking tumors of A375Pβ6 and A375Ppuro cells. Localization of labeled diabody was monitored by whole body cross-section imaging using NanoSPECT/CT. Results of these experiments showed that the αvβ6-expressing A375Pβ6 tumor was detected with the radio-labeled diabody 2h after injection and remained detectable after 5 h and 24 h (Fig. 5A, 5B). Quantification revealed significantly more radioactivity in the αvβ6-expressing tumors when compared to the A375Ppuro tumors at all three time points; the uptake was highest 5 h after injection and considerably reduced after 24 h but remained still clearly detectable (Fig. 5C). The highest normal tissue activity was found in the kidneys, a typical pattern found for radio-metal-labeled compounds due to the excretion of 99mTc-labeled compound by this organ.
Figure 5. 99mTc-labeled B6.3 diabody localised specifically to αvβ6-expressing tumors in vivo.
A) A375Pβ6 and A375Ppuro cells were injected subcutaneously on opposite shoulders and 99mTc-labeled B6.3 diabody (approximately 11 µg, 30 MBq) was injected intravenously once tumours had developed. Mice were imaged by SPECT/CT as indicated 2 h, 5 h and 24 h after injection. B) SPECT/CT cross sections of the same mice at 2, 5 and 24 h. C) Percent injected doses of 99mTc-labeled B6.3 diabody in A375Pβ6 and A375Ppuro tumours from three mice, obtained from these images. D) Biodistribution of 99mTc-labeled B6.3 diabody 24 h after injection. Data expressed as % injected dose/g (%ID/g) as mean ± SD for 5 animals. Tumor-to blood ratios at this time point were 40.4 for A375Pβ6 tumors and 15.5 for A375Ppuro tumors. Significance assessed by Student's t-test.
Twenty-four hours after injection and imaging, the mice were sacrificed and biodistribution of 99mTc-labeled diabody was determined (Fig. 5D). The data showed that %ID/g obtained in A375Pβ6 tumors was significantly higher (p<0.0005) than that in the A375Ppuro tumors, in agreement with quantification from imaging at this time point. Tumor-to-Blood ratios of 40 were obtained for the αvβ6 positive tumor whereas the αvβ6 negative tumor gave a ratio of 15.5. The kidney had the highest %ID/g of any organ in agreement with the imaging results. Imaging and biodistribution studies showed that 99mTc-labeled diabody targets specifically to αvβ6-expressing tumors in vivo and is detectable 24 h after injection with tumor-to-blood ratios suitable for imaging.
Discussion
This work describes the generation and characterization of a novel diabody that specifically targets the αvβ6 integrin. The diabody was produced as a soluble secreted protein in P. pastoris, allowing rapid production using a process readily adaptable to manufacture of clinical grade material [24], [25]. The engineered hexahistidine tag allowed purification and successful labeling with 99mTc, without affecting the nanomolar binding affinity of the diabody on cells in vitro.
Our studies also showed that the diabody inhibited adhesion and migration of the αvβ6-transfected melanoma cell line, A375Pβ6 and the pancreatic adenocarcinoma cell line, Capan-1, to LAP. This is the desired function-blocking activity of anti-αvβ6 and has been observed with whole anti-αvβ6 antibodies that block in vitro migration of αvβ6–positive Detroit 562 human pharyngeal carcinoma cells and inhibit tumor growth in vivo by suppressing TGFβ activation [16], [27]. When interactions via fibronectin, a less specific ligand, were investigated, the diabody was found to inhibit adhesion and migration of Capan-1 cells but not A375Pβ6 cells. This highlights the specificity of B6.3 because A375Pβ6 cells express other fibronectin-binding integrins [17] that would not be blocked by an αvβ6-specific agent. The αvβ6 integrin activates latent-TGFβ first by binding to LAP and then through cortical actin-dependent mechanical forces that causes distortion of the LAP molecule, releasing the TGFβ [29], [30]. Targeting αvβ6 with the B6.3 diabody inhibited this interaction with LAP, resulting in inhibition of Smad2/3 translocation to the nucleus.
The role of αvβ6-dependent TGFβ activation in cancer has been investigated in a number of studies [31], [32], [33], [34], [35], that illustrate both the potential and complexity associated with this target. For example, when αvβ6 was blocked with antibodies in the early stages of disease in a transgenic pancreatic cancer mouse model, this accelerated cancer progression when SMAD4 was functional, but not in SMAD4-null animals [34]. In separate studies αvβ6 promoted cancer growth and liver metastasis through activation of TGFβ [11], [36]. Thus, the functional blockade of αvβ6 has positive therapeutic implications due to the potential inhibition of TGFβ, although TGFβ can also act as a tumour suppressor in normal epithelium and pre-malignant transformed epithelial cells. However, cancer cells often develop mutations that prevent TGFβ-mediated growth inhibition, making TGFβ a strong tumor promoter [32], [33]. Therefore therapeutic antibody blockade of αvβ6 can suppress tumour growth [16], [35] but the molecular phenotype of the tumor must be taken into consideration.
When tested for αvβ6 localization in vivo, the radiolabeled diabody showed specific targeting of αvβ6-positive tumours, detectable as early as two hours after injection. Signal was measurable over 24 hours, although intensity was highest five hours after injection. Radiolabeling with 99mTc, using site-specific attachment to the engineered hexahistidine tag, was found to be simple and efficient. Furthermore, use of 99mTc allowed residualization of the signal within the tumor upon internalization of the diabody. These characteristics, combined with the ease of production of B6.3 and its favourable biodistribution in vivo, make this diabody an attractive tool for clinical imaging.
The diabody format has not yet been fully exploited as a cancer targeting agent, but it has many attractive features. The bivalency of diabodies conferred by their dimeric structure holds the advantage of higher tumor uptake compared to scFv fragments, resulting in higher signals when used as imaging agents [37]. Also the bigger size of diabodies in relation to scFv increases their circulatory half life, resulting in higher accumulation in the tumor, while achieving better contrast at short time points than bigger engineered fragments such as minibodies [38]. However, despite higher contrast and early imaging, radiometal-labeling of diabodies also results in considerable kidney retention, as shown in our current study and by other groups [22], [37], [38]. This can be problematic if imaging is desirable in close areas. Attachment of polyethylene glycol (PEG) to diabodies has shown to significantly lower kidney retention [22] and improvement of pharmacokinetics [21], resulting in increased circulating time that did not affect the collection of optimal images within 24 hours. The increased circulating time could in fact be advantageous if diabodies are to be used for therapeutic purposes, which required maximum tumor accumulation. In this sense, the internalization of the B6.3 could be clinically useful for delivery of toxic compounds such as the radioisotope, conjugated toxic agents should or small toxic drugs, such as pyrrolobenzodiazepines (PBDs), that are active within target cells.
In summary, the B6.3 diabody described in our study bound specifically to αvβ6 in vitro and targeted specifically to αvβ6-expressing tumors in vivo. In addition, the diabody retained the biological properties of ligand-mimicking antibodies; it showed internalization upon binding to αvβ6, successfully blocked αvβ6-dependent adhesion and migration to LAP and fibronectin and inhibited smad2/3 nuclear translocation upon treatment with latent TGFβ1. Based on its function-blocking activity and specific targeting to αvβ6-positive cells in vivo, the B6.3 diabody has potential as an imaging agent or a building block for generation of therapeutics by chemical coupling of small cytotoxic molecules or addition of toxic agents.
Acknowledgments
The authors thank Hector Knight (Covidien) for providing the Isolink kits. We are also grateful to Professor Ian Hart for his valuable intellectual suggestions in this study.
Funding Statement
This study was supported by Debbie Fund and the UCL Cancer Institute Research Trust, Cancer Research UK (grant number C34/A5149), the Department of Health and Cancer Research UK Experimental Cancer Medicine Centre (grant number C34/A7279) (www.cancerresearchuk.org) (www.ecmcnetwork.org.uk) and the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) (ref. UCLH.BW.mn.10161 (http://www.uclhospitals.brc.nihr.ac.uk). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Patsenker E, Wilkens L, Banz V, Osterreicher CH, Weimann R, et al. (2010) The alphavbeta6 integrin is a highly specific immunohistochemical marker for cholangiocarcinoma. J Hepatol 52: 362–369. [DOI] [PubMed] [Google Scholar]
- 2. Bandyopadhyay A, Raghavan S (2009) Defining the role of integrin alphavbeta6 in cancer. Curr Drug Targets 10: 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sipos B, Hahn D, Carceller A, Piulats J, Hedderich J, et al. (2004) Immunohistochemical screening for beta6-integrin subunit expression in adenocarcinomas using a novel monoclonal antibody reveals strong up-regulation in pancreatic ductal adenocarcinomas in vivo and in vitro. Histopathology 45: 226–236. [DOI] [PubMed] [Google Scholar]
- 4. Bates RC (2005) Colorectal cancer progression: integrin alphavbeta6 and the epithelial-mesenchymal transition (EMT). Cell Cycle 4: 1350–1352. [DOI] [PubMed] [Google Scholar]
- 5. Hazelbag S, Kenter GG, Gorter A, Dreef EJ, Koopman LA, et al. (2007) Overexpression of the alpha v beta 6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J Pathol 212: 316–324. [DOI] [PubMed] [Google Scholar]
- 6. Thomas GJ, Nystrom ML, Marshall JF (2006) Alphavbeta6 integrin in wound healing and cancer of the oral cavity. J Oral Pathol Med 35: 1–10. [DOI] [PubMed] [Google Scholar]
- 7. Shi M, Zhu J, Wang R, Chen X, Mi L, et al. (2011) Latent TGF-beta structure and activation. Nature 474: 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thomas GJ, Lewis MP, Hart IR, Marshall JF, Speight PM (2001) AlphaVbeta6 integrin promotes invasion of squamous carcinoma cells through up-regulation of matrix metalloproteinase-9. Int J Cancer 92: 641–650. [DOI] [PubMed] [Google Scholar]
- 9. Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, et al. (1999) The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96: 319–328. [DOI] [PubMed] [Google Scholar]
- 10. Zhang ZY, Xu KS, Wang JS, Yang GY, Wang W, et al. (2008) Integrin alphanvbeta6 acts as a prognostic indicator in gastric carcinoma. Clin Oncol (R Coll Radiol) 20: 61–66. [DOI] [PubMed] [Google Scholar]
- 11. Yang GY, Xu KS, Pan ZQ, Zhang ZY, Mi YT, et al. (2008) Integrin alpha v beta 6 mediates the potential for colon cancer cells to colonize in and metastasize to the liver. Cancer Sci 99: 879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mamuya FA, Duncan MK (2012) aV integrins and TGF-beta-induced EMT: a circle of regulation. J Cell Mol Med 16: 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bates RC, Mercurio AM (2005) The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biol Ther 4: 365–370. [DOI] [PubMed] [Google Scholar]
- 14. Singh A, Greninger P, Rhodes D, Koopman L, Violette S, et al. (2009) A gene expression signature associated with “K-Ras addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell 15: 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang SB, Du Y, Wu BY, Xu SP, Wen JB, et al. (2012) Integrin alphavbeta6 promotes tumor tolerance in colorectal cancer. Cancer Immunol Immunother 61: 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Aarsen LA, Leone DR, Ho S, Dolinski BM, McCoon PE, et al. (2008) Antibody-mediated blockade of integrin alpha v beta 6 inhibits tumor progression in vivo by a transforming growth factor-beta-regulated mechanism. Cancer Res 68: 561–570. [DOI] [PubMed] [Google Scholar]
- 17. Kogelberg H, Tolner B, Thomas GJ, Di Cara D, Minogue S, et al. (2008) Engineering a single-chain Fv antibody to alpha v beta 6 integrin using the specificity-determining loop of a foot-and-mouth disease virus. J Mol Biol 382: 385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holliger P, Prospero T, Winter G (1993) “Diabodies”: small bivalent and bispecific antibody fragments. Proc Natl Acad Sci U S A 90: 6444–6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu AM, Yazaki PJ (2000) Designer genes: recombinant antibody fragments for biological imaging. Q J Nucl Med 44: 268–283. [PubMed] [Google Scholar]
- 20. Adams GP, Schier R, McCall AM, Crawford RS, Wolf EJ, et al. (1998) Prolonged in vivo tumour retention of a human diabody targeting the extracellular domain of human HER2/neu. Br J Cancer 77: 1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holliger P, Hudson PJ (2005) Engineered antibody fragments and the rise of single domains. Nat Biotechnol 23: 1126–1136. [DOI] [PubMed] [Google Scholar]
- 22. Li L, Turatti F, Crow D, Bading JR, Anderson AL, et al. (2010) Monodispersed DOTA-PEG-conjugated anti-TAG-72 diabody has low kidney uptake and high tumor-to-blood ratios resulting in improved 64Cu PET. J Nucl Med 51: 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DiCara D, Rapisarda C, Sutcliffe JL, Violette SM, Weinreb PH, et al. (2007) Structure-function analysis of Arg-Gly-Asp helix motifs in alpha v beta 6 integrin ligands. J Biol Chem 282: 9657–9665. [DOI] [PubMed] [Google Scholar]
- 24. Tolner B, Smith L, Begent RH, Chester KA (2006) Expanded-bed adsorption immobilized-metal affinity chromatography. Nat Protoc 1: 1213–1222. [DOI] [PubMed] [Google Scholar]
- 25. Tolner B, Smith L, Begent RH, Chester KA (2006) Production of recombinant protein in Pichia pastoris by fermentation. Nat Protoc 1: 1006–1021. [DOI] [PubMed] [Google Scholar]
- 26. Graff CP, Chester K, Begent R, Wittrup KD (2004) Directed evolution of an anti-carcinoembryonic antigen scFv with a 4-day monovalent dissociation half-time at 37 degrees C. Protein Eng Des Sel. 17: 293–304. [DOI] [PubMed] [Google Scholar]
- 27. Weinreb PH, Simon KJ, Rayhorn P, Yang WJ, Leone DR, et al. (2004) Function-blocking integrin alphavbeta6 monoclonal antibodies: distinct ligand-mimetic and nonligand-mimetic classes. J Biol Chem 279: 17875–17887. [DOI] [PubMed] [Google Scholar]
- 28. He J, Wang Y, Feng J, Zhu X, Lan X, et al. (2010) Targeting prostate cancer cells in vivo using a rapidly internalizing novel human single-chain antibody fragment. J Nucl Med 51: 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Giacomini MM, Travis MA, Kudo M, Sheppard D (2012) Epithelial cells utilize cortical actin/myosin to activate latent TGF-beta through integrin alpha(v)beta(6)-dependent physical force. Exp Cell Res 318: 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sheppard D (2003) Functions of pulmonary epithelial integrins: from development to disease. Physiol Rev 83: 673–686. [DOI] [PubMed] [Google Scholar]
- 31. Roberts AB, Wakefield LM (2003) The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A 100: 8621–8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meulmeester E, Ten Dijke P (2011) The dynamic roles of TGF-beta in cancer. J Pathol 223: 205–218. [DOI] [PubMed] [Google Scholar]
- 33. Elliott RL, Blobe GC (2005) Role of transforming growth factor Beta in human cancer. J Clin Oncol 23: 2078–2093. [DOI] [PubMed] [Google Scholar]
- 34. Hezel AF, Deshpande V, Zimmerman SM, Contino G, Alagesan B, et al. (2012) TGF-beta and alphavbeta6 integrin act in a common pathway to suppress pancreatic cancer progression. Cancer Res 72: 4840–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eberlein C, Kendrew J, McDaid K, Alfred A, Kang JS, et al.. (2012) A human monoclonal antibody 264RAD targeting alphavbeta6 integrin reduces tumour growth and metastasis, and modulates key biomarkers in vivo. Oncogene. [DOI] [PubMed]
- 36. Lee MS, Kim TY, Kim YB, Lee SY, Ko SG, et al. (2005) The signaling network of transforming growth factor beta1, protein kinase Cdelta, and integrin underlies the spreading and invasiveness of gastric carcinoma cells. Mol Cell Biol 25: 6921–6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu AM, Olafsen T (2008) Antibodies for molecular imaging of cancer. Cancer J 14: 191–197. [DOI] [PubMed] [Google Scholar]
- 38. Sundaresan G, Yazaki PJ, Shively JE, Finn RD, Larson SM, et al. (2003) 124I-labeled engineered anti-CEA minibodies and diabodies allow high-contrast, antigen-specific small-animal PET imaging of xenografts in athymic mice. J Nucl Med 44: 1962–1969. [PMC free article] [PubMed] [Google Scholar]