Abstract
Our objective was to test the hypothesis that fetal urine contains a substance(s) that regulates amniotic fluid volume by altering the rate of intramembranous absorption of amniotic fluid. In late gestation ovine fetuses, amniotic fluid volumes, urine, and lung liquid production rates, swallowed volumes and intramembranous volume and solute absorption rates were measured over 2-day periods under control conditions and when urine was removed and continuously replaced at an equal rate with exogenous fluid. Intramembranous volume absorption rate decreased by 40% when urine was replaced with lactated Ringer solution or lactated Ringer solution diluted 50% with water. Amniotic fluid volume doubled under both conditions. Analysis of the intramembranous sodium and chloride fluxes suggests that the active but not passive component of intramembranous volume absorption was altered by urine replacement, whereas both active and passive components of solute fluxes were altered. We conclude that fetal urine contains an unidentified substance(s) that stimulates active intramembranous transport of amniotic fluid across the amnion into the underlying fetal vasculature and thereby functions as a regulator of amniotic fluid volume.
Keywords: kidney, polyhydramnios
in the latter half of gestation, the volume of amniotic fluid is determined by a balance of four fluids that flow into and out of the amniotic sac. Previous investigations in experimental animals have shown conclusively that three of these flows, urine production, swallowed volume, and lung liquid secretion, are regulated primarily to meet fetal body needs and thus only affect or modify but do not regulate amniotic fluid volume (6, 7, 16, 20, 22). In contrast, intramembranous absorption of amniotic fluid across the amnion and into the underlying fetal vasculature has been shown to be actively regulated to maintain amniotic fluid volume near normal under several experimental conditions (2, 7, 9, 10, 14, 19, 22). Thus an understanding of the mechanisms that regulate amniotic fluid volume requires knowledge of the factors involved in regulating intramembranous absorption.
The factors that participate in the regulation of intramembranous absorption of amniotic fluid are currently not known. Stretch of the fetal membranes has been examined. However, studies in pregnant sheep found that experimentally induced stretch of the amniotic membranes over several days by inflating and deflating balloons placed in the amniotic fluid was without effect on the volume of amniotic fluid despite significant changes in the total intrauterine volume (10).
Constituents of the amniotic fluid may act as regulators of intramembranous absorption. There are four potential sources of such regulatory factors: fetal urine, fetal lung liquid, fetal membranes, and fetal oral-nasal secretion. Previous studies speculated that fetal lung liquid may contain a substance that could alter the rate of intramembranous absorption. However, recent studies have shown that the fetal lung liquid that enters the amniotic fluid was without effect on the passive and active components of intramembranous absorption (12, 15, 19). Because fetal urine is a principal source of amniotic fluid and a large volume of urine enters the amniotic cavity each day, we hypothesized that fetal urine is a source of a chemical substance(s) that enters the amniotic fluid to act as a regulator of intramembranous absorption. To test this hypothesis, we measured amniotic fluid volume and intramembranous absorption rate under normal conditions of fetal urine entry into the amniotic fluid and when fetal urine was continuously removed and isovolumically replaced with exogenous fluids.
METHODS
Ethical approval.
All experimental and surgical procedures were approved by the Institutional Animal Care and Use Committee at Oregon Health and Science University. The experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (National Research Council).
Surgical procedures.
Eight time-bred ewes were obtained from a local breeder. Fetal catheterizations were performed at 120 days of gestation (term ∼ 145 days). Sheep were denied food before surgery but had free access to water. To induce anesthesia, the ewe was given an intramuscular injection of atropine (7.5 mg), followed by an intravenous injection of 400 mg ketamine and 10 mg diazepam. After intubation, anesthesia was maintained with a mixture of oxygen and nitrous oxide (3:1) and 1–2% isoflurane. This regimen fully anesthetizes the fetus.
The fetal surgical procedures have been described elsewhere (19). Briefly, catheters were placed in a fetal carotid artery and jugular vein. A large bore catheter was placed with one end in the trachea and the other end attached to the fetal skin, allowing continuous flow of lung liquid from the fetal lungs into the amniotic cavity. The center loop of this catheter was exteriorized, providing access to the lung fluid at the time of the experiment. A flow sensor (Transonic Systems; Ithaca, NY) was placed around the mid cervical esophagus. Multiple intra-amniotic catheters were attached to the fetal skin for measurement of amniotic fluid pressure, return of fetal lung liquid and fetal urine to the amniotic sac, infusion of exogenous fluid or drainage of amniotic fluid. A catheter with a Silastic tip was placed in the fetal bladder. The urachus was ligated, and the fetal incisions were repaired. The membranes and uterine incision were carefully repaired to ensure approximation of the fetal membranes and a water-tight closure of the membranes and uterus. The vascular catheters were filled with heparinized saline. The catheters and flow sensor cable were routed under the maternal skin, exiting on her flank, and stored in a pouch attached to the skin of the ewe. One million units of penicillin G and 2 mg of ciprofloxacin were injected into the amniotic fluid. Postsurgery, each ewe received 0.6 mg buprenorphine for 2 days for analgesia.
Experimental procedures.
Four or five days after surgery, the ewe was brought to the laboratory and placed in a cage with free access to food and water. The fetal esophageal flow sensor was connected to a Transonic flow meter and continuous swallowing data were recorded for the duration of the experiment by means of an ADInstruments PowerLab (Colorado Springs, CO). Urine and lung liquid were drained continuously to the exterior for measurement of flow rates and returned to the amniotic compartment as previously described (19). The experiment began on the following day.
Each fetus took part in a control protocol and at least one of two experimental protocols. The control protocol consisted of continuous drainage and return of urine to the amniotic sac. The experimental protocols consisted of continuous drainage of the urine and its replacement with an equal flow of one of two sterile solutions: lactated Ringer solution or lactated Ringer solution diluted 50% with water. The fetuses were randomly assigned to each protocol. Randomization was performed to eliminate fetal age and body weight as factors in the observed responses.
At the completion of the experiments, the ewes and fetuses were killed by an intravenous injection of euthanasia solution (Euthasol Solution), as approved by the Institutional Animal Care and Use Committee. The membranes were carefully examined to ensure that they were free of holes or tears. The positioning of the fetal catheters and the flow sensor was confirmed, and fetal weights were determined.
Measurements.
To initiate an experimental protocol, amniotic fluid was drained from the uterus through the large bore catheters to measure amniotic fluid volume (9, 10, 19). After drainage, 1 liter of lactated Ringer solution at 39°C was returned to the uterus, allowing all protocols to start with the same volume and composition of fluid in the amniotic compartment. Each protocol lasted ∼48 h. At the completion of each protocol, arterial blood gases and pH, blood pressures, heart rate, and amniotic fluid volume were measured.
Arterial blood pH, Pco2, Po2, hematocrit, and oxygen content were analyzed with a Radiometer ABL 725 analyzer (Copenhagen, Denmark) and the values corrected to fetal body temperature (39°C). Fetal blood and amniotic fluid concentrations of sodium and chloride were measured with the Radiometer analyzer. Hydrostatic pressures were measured using pressure transducers (Abbott Transpac; Abbott Park, IL) and recorded using PowerLab (ADInstruments). Intravascular pressures are reported with respect to amniotic fluid pressure. Heart rates were derived from the arterial pressure pulsations. Osmolality of the exogenous fluids was measured with an Advanced Instruments model 3D3 osmometer (Norwood, MA). Esophageal flow was recorded continuously using a T106 small animal flowmeter (Transonic Systems). The volume of swallowed fluid was determined by integrating the area under the esophageal flow curve (19).
The 2-day average intramembranous absorption rate was calculated from the difference between the time integrated amniotic sac inflows (urine or its replacement and lung liquid), outflow (swallowing), and the change in amniotic fluid volume over the duration of each protocol (19). The average intramembranous fluxes of sodium and chloride were calculated similarly by determining the difference between the initial and final amniotic solute masses (mass = volume × concentration) and adding the solute inflows while subtracting solute outflow due to swallowing (12). Solute absorption from amniotic fluid to fetus was considered positive, whereas that in the opposite direction was considered negative. It was assumed that there were no fluxes across the uterine wall between the ewe and the amniotic fluid (1).
Data interpretation.
Previous studies have shown that intramembranous volume absorption has two components: a primary bulk flow component with dissolved amniotic solutes that is directed outward from the amniotic compartment to fetal blood and a smaller, secondary component due to bidirectional movement through water channels (wc) (5, 8, 12, 14). The net intramembranous volume absorption (IMA) can be expressed as
| (1) |
The intramembranous transport of dissolved solutes such as sodium or chloride also has been shown to have two components: 1) a primary, active bulk component that contains amniotic fluid and is directed outward from the amniotic fluid and 2) a smaller, passive diffusional component that is dependent on intramembranous solute permeability (P) and the solute concentration difference (ΔC) between amniotic fluid (af) and fetal blood that can move bidirectionally (5, 12). The intramembranous flux of solute s (Sols) can be expressed as
| (2) |
The latter equation assumes that passive solute fluxes occur independently of passive volume flow, as suggested by previous studies (5, 12). These two equations are used for interpretation of the observed experimental data.
Statistics.
Differences between group means were compared using Student's paired t-test. Comparisons of arterial blood gas data and hemodynamic data were made between data collected on the initial day of the experiment and the final day of the experiment. All other comparisons were for data collected under control conditions and data collected either after urine replacement with lactated Ringer solution or urine replacement with diluted lactate Ringer solution, except as noted in Table 1. Bivariate least squares linear regression was used to determine the slope and intercept of the regression relationship between two variables. Relationships between intramembranous solute flux and volume absorption rates were compared with a one factor analysis of covariance (ANCOVA). To determine whether the slopes were parallel, the ANCOVA was repeated after subtracting the Y-axis intercept from the solute fluxes under each of the three experimental conditions. One animal was excluded from the solute flux analyses because electrolyte concentrations were not available. Amniotic fluid-to-fetal solute concentration differences were compared with a one factor analysis of variance (ANOVA). Fisher's least significant difference was used for post hoc testing when the null hypothesis was rejected. Differences were considered statistically significant at P < 0.05. Data are reported as means ± SE.
Table 1.
Comparison of responses to urine diversion and replacement with lactated Ringer solution or lactated Ringer solution diluted by 50% with water
| Control (n = 8) | LR (n = 8) | Control vs. LR P | Dilute LR (n = 5) | LR vs. dilute LR P | |
|---|---|---|---|---|---|
| Amniotic fluid volume, ml | 857 ± 172 | 1,537 ± 245 | 0.007 | 1,345 ± 160 | NS |
| IA flow, ml/h | 35 ± 4 | 20 ± 6 | 0.01 | 22 ± 8 | NS |
| Swallowing flow, ml/h | 27 ± 4 | 34 ± 4 | NS | 44 ± 10 | NS |
| Urine flow, ml/h | 44 ± 5 | 47 ± 7 | NS | 50 ± 4 | NS |
| Lung liquid flow, ml/h | 16 ± 3 | 18 ± 4 | NS | 24 ± 2 | 0.02 |
Data reported as means ± SE; n, number of fetuses. LR, lactated Ringer solution; IA, intramembranous absorption; NS not significant. Comparisons were made between data measured during the control period and after urine diversion with continual replacement using lactated Ringer solution or between data measured after urine diversion with continual replacement with lactated Ringer solution and continual replacement with dilute lactated Ringer solution. Experimental periods were performed in a random order and each period lasted approximately 48 h. All comparisons were made using paired t-test.
RESULTS
Amniotic fluid volume and intramembranous absorption responses to fetal urine replacement.
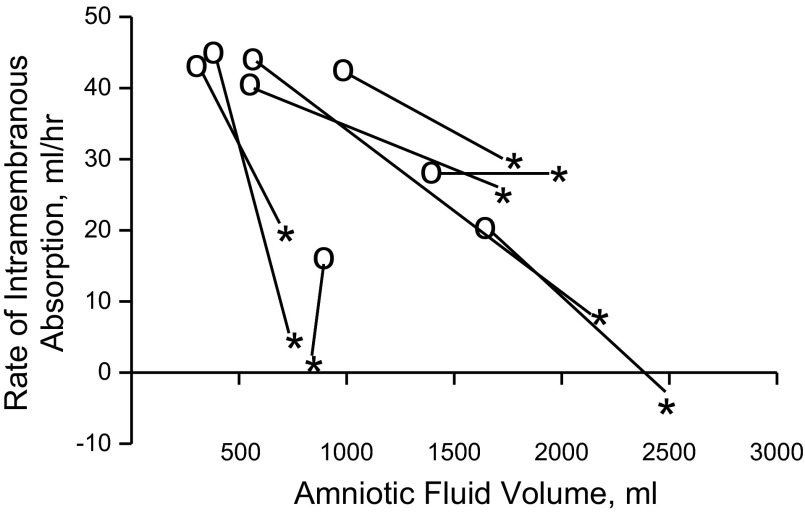
Diversion of fetal urine away from the amniotic fluid and its isovolumic replacement with lactated Ringer solution resulted in a decrease in the rate of intramembranous absorption and an increase in amniotic fluid volume (Fig. 1). The experimental results from each protocol are reported in Table 1. When compared with control, replacement of fetal urine with lactated Ringer solution resulted in a near doubling of amniotic fluid volume and a decrease in the rate of intramembranous absorption to 60% of the control value (Table 1). There were no statistically significant changes in any of the other measured fetal flows.
Fig. 1.
Response of amniotic fluid volume and the rate of intramembranous absorption to diversion of fetal urine away from the amniotic fluid and isovolumic replacement with lactated Ringer solution. Isovolumic replacement of fetal urine resulted in an increase in amniotic fluid volume and a drop in the rate of intramembranous absorption. Open circles represent the control conditions and asterisks represent data collected during 2 days of urine diversion with isovolumic replacement by lactated Ringer solution. N = 8 fetuses.
In 5 of 8 animals, fetal urine was replaced by lactated Ringer solution diluted 50% with sterile water. With the exception of a small increase in the volume of lung liquid over the 2-day period (P = 0.02, paired t-test), there were no differences in any of the measured amniotic inflows and outflows between these five fetuses and those that received undiluted Ringer solution for urine replacement (Table 1).
Intramembranous sodium and chloride fluxes and concentration differences.
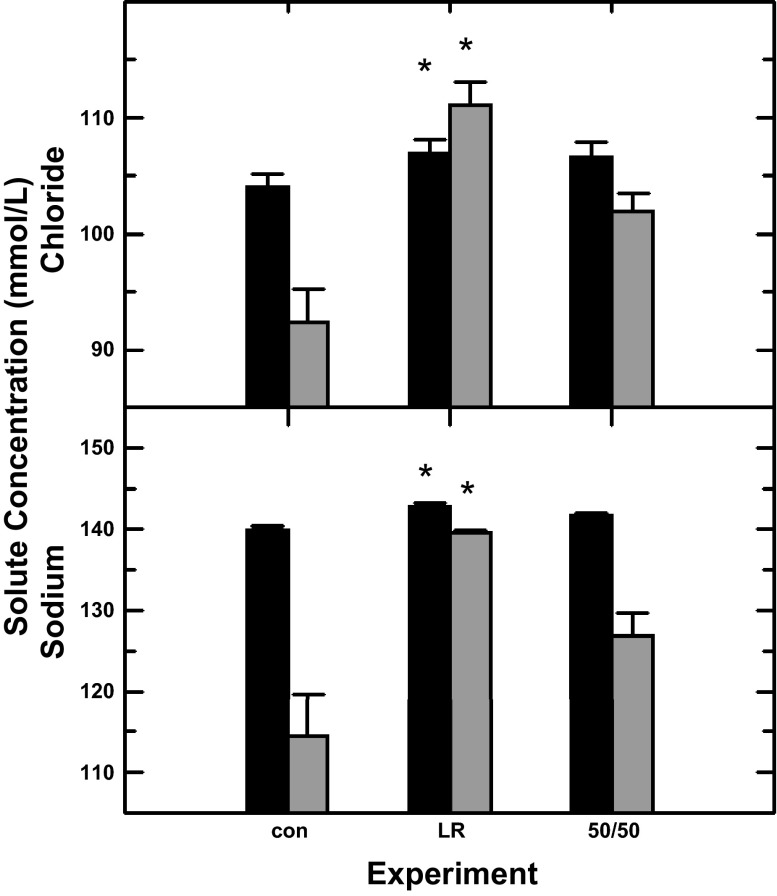
Fetal blood and amniotic fluid sodium and chloride concentrations were statistically increased (P < 0.05) during fetal urine replacement with lactated Ringer solution. While there was a tendency for increases during replacement with the diluted lactated Ringer solution, the increases were not statistically significant. Urine replacement with lactated Ringer solution but not diluted Ringer solution resulted in reductions (P < 0.01) in the amniotic fluid-to-fetal plasma concentration differences (Fig. 2).
Fig. 2.
Amniotic fluid (shaded bars) and fetal blood (solid bars) sodium and chloride concentrations as a function of experimental condition: con, control with urine entering the amniotic sac (n = 7); LR, fetal urine replaced with lactated Ringer solution (n = 7); 50/50, urine replaced with a solution of lactated Ringer diluted by 50% with water (n = 5). Results were analyzed by paired t-test comparing the control group (n = 7) to the group receiving lactated Ringer solution or comparing the control group (n = 5) to the group receiving dilute lactated Ringer solution. *P < 0.05 compared with control values.
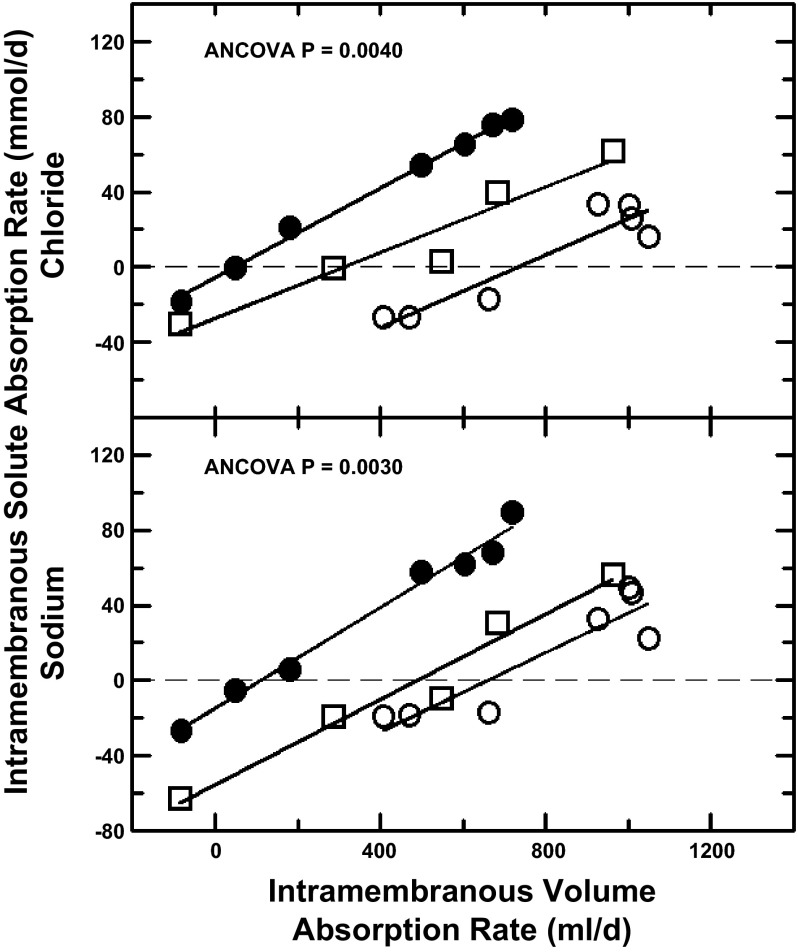
Intramembranous sodium and chloride flux rates were linearly related to intramembranous volume absorption rate both under control conditions with return of fetal urine to the amniotic fluid and with isovolumic replacement of fetal urine with lactated Ringer solution. However, urine replacement resulted in highly significant leftward shifts in the curves relating solute flux rate to intramembranous volume absorption rate (Fig. 3). When undiluted or 50% diluted lactated Ringer solution entered the amniotic fluid, the leftward shifts were due to changes in solute concentrations as the slopes of the regression equations were unchanged. Furthermore, the slopes (117.7 ± 8.7 mmol/l for sodium and 100.7 ± 9.4 mmol/l for chloride) were not significantly different from the mean amniotic fluid sodium (127.0 ± 12.6 mmol/l) or chloride (101.8 ± 9.4 mmol/l) concentrations. Intercepts of the three regression lines (when volume flux = 0) divided by the respective mean amniotic fluid-to-fetal blood concentration differences (Eq. 2) yielded estimates for intramembranous sodium permeability of 3.7 ± 0.5 l/day and chloride permeability of 4.4 ± 1.6 l/day.
Fig. 3.
Intramembranous sodium and chloride absorption rates as functions of intramembranous volume absorption rate during control conditions and when fetal urine was diverted from the amniotic sac and replaced with exogenous fluids. Open circles, control conditions; filled circles, fetal urine continuously and isovolumically replaced with lactated Ringer solution; open squares, urine replaced with lactated Ringer solution diluted by 50% with water. Solid lines, regression lines from the ANCOVA. Isovolumic urine replacement resulted in a leftward shift for both chloride (P = 0.0040) and sodium (P = 0.0030).
Intramembranous absorption and the fetal-amniotic sodium gradient.
The relationship between fluid movement and the sodium gradient between the fetus and amniotic fluid was examined. Regression analysis of the rate of intramembranous absorption versus the fetal-to-amniotic fluid sodium gradient was performed for each of the three experimental protocols (control, urine replacement with lactated Ringer solution, and urine replacement with 50/50 lactated Ringer solution). Intramembranous absorption rate and sodium gradient were not related statistically for any protocol. Furthermore, for all data combined, there was no significance.
Fetal blood gas and cardiovascular values.
Fetal blood gas compositions are reported in Table 2. There were no statistically significant differences between the initial and the final values for any variable. Initially, arterial blood pressure was 45 ± 2 mmHg, venous blood pressure was 3.1 ± 0.5 mmHg, and heart rate was 179 ± 8 beats/min. At the end of experimentation, arterial blood pressure was 47 ± 2 mmHg, venous blood pressure was 2.6 ± 0.6 mmHg, and heart rate was 164 ± 7 beats/min. None of these changes was statistically significant.
Table 2.
Fetal arterial blood values at beginning and end of experimentation
| Initial Experiment | Final Experiment | |
|---|---|---|
| pH | 7.357 ± 0.010 | 7.354 ± 0.014 |
| Pco2, mmHg | 52.9 ± 1.4 | 52.5 ± 0.8 |
| Po2, mmHg | 21.5 ± 0.8 | 20.9 ± 1.0 |
| Hematocrit, % | 31 ± 1 | 31 ± 1 |
| Oxygen content, ml/dl | 7.6 ± 0.3 | 7.5 ± 0.3 |
Data are reported as means ± SE; there were no statistically significant differences between any values measured comparing the initial and final experimental protocols using Student's paired t-test. Measurements were made on arterial blood samples from 7 fetuses. Because of catheter failure, jugular vein blood samples were analyzed in the 8th fetus with the following results: Initial experiment: pH = 7.322; Pco2 = 63.0 mmHg; Po2 = 16.9 mmHg; hematocrit = 32%; oxygen content = 5.7 ml/dl. Final experiment: pH = 7.337; Pco2 = 59.5 mmHg; Po2 = 18.3 mmHg; hematocrit = 36%; oxygen content = 6.6 ml/dl.
Autopsy.
Amniochorionic membrane integrity was confirmed in all experiments as was the correct placement of all instrumentation. The average fetal weight at autopsy was 4.52 ± 0.10 kg at 136 ± 1 days of gestation.
DISCUSSION
The results of this study suggest that constituents in fetal urine play a significant role in regulating amniotic fluid volume because continuous, isovolumic replacement of fetal urine with lactated Ringer solution, either diluted or undiluted, altered both amniotic fluid volume and the rate of intramembranous absorption. From the fact that past studies failed to find an effect of fetal membrane stretch (10) or fetal lung liquid constituents (12, 15, 19) on intramembranous absorption rate, the present observation that intramembranous absorption rate decreases in response to fetal urine replacement is important because it provides for the first time an indication that regulatory factors may be the primary mediators of amniotic fluid volume regulation, at least in late gestation. The rate of delivery of any urinary mediator would be dependent on both the concentration of the mediator in the urine and the rate of urine flow. Based on past studies, fetal urine constituents are not likely to be the only regulator(s) of intramembranous absorption rate. First, the decrease in amniotic fluid volume following embolization of the fetal side of the placental is not associated with changes in fetal urine flow rate (11). Second, fluid infusion into the amniotic compartment is associated with an increase in intramembranous absorption rate (4, 9, 13, 14, 19) that may be independent of increases in urine flow rate. While it has been demonstrated by Tomoda and colleagues (23) that amniotic fluid volume is linearly related to fetal weight, this would not be a consideration in these experiments because the sequence of the experimental protocols was randomized.
In the present study, the volume and flow measurements were made 2 days after the start of urine replacement. We have previously shown in sheep that, following ligation of the fetal esophagus, amniotic fluid volume increased progressively over 9 days (15). Computer simulations (3) suggest that several days may be required to establish steady-state conditions following a change in amniotic inflows or outflows. Thus an increase in amniotic fluid volume beyond 2 days to levels approximating polyhydramnios may occur with sustained reductions in intramembranous absorption rate over time. Another consideration in solute data interpretation is that the calculated solute flux rates were based on amniotic fluid, urine, and lung liquid concentrations at the start and end of the experimental period rather than average values over time. This method of data analysis would increase the experimental error in the calculated solute flux rates. However, the very tight correspondence of solute flux rate with intramembranous volume absorption rate (Fig. 3) suggests that any error is minimal.
To decipher the dynamic changes in fluid flows observed in this study, it is important to consider whether the decrease in intramembranous volume absorption rate and the associated increase in amniotic fluid volume was attributed to unidentified substances in fetal urine or to the difference in osmolality and/or concentrations of major fetal urine constituents compared with the urine replacement solutions used. We chose lactated Ringer solution as a urine replacement based on several previous experimental observations. First, infusion of either lactated Ringer solution or amniotic fluid into ovine amniotic cavity yielded essentially identical changes in amniotic fluid volume over time (4). Also, diversion of fetal lung liquid secretions that normally enter the amniotic fluid and replacement with lactated Ringer solution altered neither amniotic fluid volume nor intramembranous absorption rate (19). Finally, infusion of concentrated sodium lactate into the amniotic fluid for 3 days did not alter amniotic fluid volume (21). Collectively, these studies suggest that neither amniotic fluid volume nor intramembranous absorption rate is altered by lactated Ringer solution or lactate. Analysis of the sodium flux with sodium concentration gradient also did not show a difference.
Another important point is that the osmolality of fetal sheep urine in late gestation has been shown to range from a mean of 134 to 177 mosmol/kg water (4, 17, 18) or roughly half of the 254 mosmol/kg of the lactated Ringer solution. To take into consideration the osmolality differences between fetal urine and lactated Ringer solution, we evaluated the intramembranous absorption response to replacement of urine with either 100% or 50% lactated Ringer solution. Osmolality of the Ringer diluted 50% with water (127 mosmol/kg) should mimic that of fetal urine. Since there were no differences in the response of the relevant pathways that contribute to amniotic fluid volume regulation between the two replacement solutions, it is apparent that the large decreases in intramembranous volume absorption during urine replacement were not osmotically induced but instead were mediated by a yet-to-be-identified substance in fetal urine. This finding is consistent with a previous study suggesting that the osmotic component of intramembranous absorption is small under basal conditions (5).
Earlier studies have repeatedly shown intramembranous volume absorption to increase when amniotic fluid volume was experimentally elevated and urine flow increased (2, 7, 9, 19). In contrast, the present study revealed a decrease in intramembranous volume absorption even though amniotic fluid volume doubled while urine flow was unchanged. This decrease in absorption rate during isovolumic urine replacement while amniotic fluid volume increased is opposite to that expected and thus provides strong evidence in support of the concept that fetal urine contains stimulators of intramembranous absorption. In four of eight fetuses (Fig. 1), intramembranous volume absorption rates were near zero during urine replacement even in the presence of adequate amounts of amniotic fluid. While it is possible that other pathways besides lung liquid and urine could contribute to amniotic fluid volume, it is unlikely. Rather we consider this observation as further evidence for the existence of a biochemical factor in fetal urine that is capable of altering intramembranous absorption.
Past studies have shown that intramembranous absorption consists of at least three components (5, 8, 12, 14): 1) an active unidirectional bulk transfer of amniotic water with dissolved solutes moving outward across the amnion into fetal blood; 2) passive diffusion of solutes down concentration gradients with sodium and chloride diffusing from fetal blood into the amniotic fluid; and 3) flow of water from the amniotic compartment into fetal blood presumably through aquaporin water channels driven by the osmotic gradient between amniotic fluid and fetal blood, with amniotic fluid osmolality lower than fetal blood. Each of these three components could have been altered during fetal urine replacement. However, urine replacement with lactated Ringer diluted 50% with water had essentially the same effect on intramembranous volume absorption as with undiluted Ringer solution even though sodium concentration differed, suggesting that most of the changes in intramembranous volume absorption during urine replacement were not osmotically mediated but instead were due to changes in the outwardly directed active bulk transport. This is consistent with previous studies suggesting that osmotic water flow through water channels in sheep is small compared with a large, unidirectional, outward bulk transport of amniotic water along with solutes (5, 12).
As shown by comparison between Eqs. 1 and 2, a large osmotic flow of water would be expected to yield slopes of the solute flux curves versus intramembranous volume absorption rates that are lower than the amniotic solute concentrations. However, the slopes of the solute flux curves equaled the amniotic fluid sodium and chloride concentrations. This implies that essentially all of the water passing through the intramembranous pathway was transporting sodium and chloride at the same concentration as was present in the amniotic fluid so that flow through the water channel would be minimal. The latter conclusion is consistent with the concept that experimentally induced changes in intramembranous transport of water and solutes are due to alterations in the active, outwardly directly bulk component of intramembranous transport with little or no change in the passive components (5, 12).
With respect to passive diffusion of solutes, the vertical axes intercepts for intramembranous solute absorption rate shown in Fig. 3 were reduced when the amniotic-to-fetal concentration differences were reduced (Fig. 2). These changes in passive fluxes would be expected from the changes in the solute concentrations (Eq. 2) if the passive (diffusional) permeability characteristics of the intramembranous pathway were unchanged during fetal urine replacement. The leftward shift in the solute flux curves during urine replacement suggests that the active bulk component of intramembranous solute absorption was altered during fetal urine replacement but does not suggest changes in the passive permeability characteristics as the curves were parallel. It is also noteworthy that approximately half of the net intramembranous solute fluxes (Fig. 3) under each of the three experimental conditions was directed from fetal blood into amniotic fluid. This occurred when intramembranous volume absorption rates were low and shows that the passive diffusion of sodium and chloride could make a large contribution to the amniotic fluid concentration of the solutes.
In conclusion, our studies demonstrated that fetal urine is a source of a factor(s) that stimulates intramembranous absorption because removal of urinary input to amniotic fluid results in reductions in intramembranous absorption rate, leading to increases in amniotic fluid volume. Further studies will be needed to determine the identity of this factor and elucidate the activator of this substance.
Perspectives and Significance
An understanding of the mechanisms that regulate amniotic fluid volume has long been sought because this knowledge would provide a scientific basis for the development of therapeutic approaches for treating the one-half million cases of oligohydramnios and polyhydramnios that occur in the United States each year. Unlike the normal variations shown in experimental animals, such increased or reduced volumes occur under pathological conditions when amniotic fluid volumes are beyond clinically accepted volumes. Potential benefits of effective treatments would include reduced pregnancy loss, improved perinatal outcome, and better long-term health of the mother and newborn. Although previous studies repeatedly demonstrated that amniotic fluid volume is actively regulated under many experimental conditions, those studies have failed to show the mechanisms by which the regulation occur. The significance of the present study is that it clearly demonstrates the presence of a chemical substance(s) in fetal urine that acts as a primary regulator of intramembranous absorption rate and hence of amniotic fluid volume. This is the first significant step toward unravelling the mechanisms that regulate intramembranous absorption rate. The identity of this substance and the associated regulatory pathways await future studies.
GRANTS
These experiments could not be performed without the financial support of National Institutes of Health Grants HD-061541 and HD-034430 as well as the technical assistance of Loni Socha, Robert Webber, and Elizabeth Clarke.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.F.A., C.Y.C., and R.A.B. conception and design of research; D.F.A., S.S.J., S.L., C.Y.C., and R.A.B. performed experiments; D.F.A. and R.A.B. analyzed data; D.F.A., S.S.J., and R.A.B. interpreted results of experiments; D.F.A. and R.A.B. prepared figures; D.F.A. and R.A.B. drafted manuscript; D.F.A., S.S.J., S.L., C.Y.C., and R.A.B. edited and revised manuscript; D.F.A., S.S.J., S.L., C.Y.C., and R.A.B. approved final version of manuscript.
REFERENCES
- 1.Anderson DF, Borst NJP, Boyd RDH, Faber JJ. Filtration of water from mother to conceptus via paths independent of fetal placental circulation in sheep. J Physiol 431: 1–10, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brace RA. Fetal blood volume, urine flow, swallowing, and amniotic fluid volume responses to long-term intravascular infusions of saline. Am J Obstet Gynecol 161: 1049–1054, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Brace RA, Anderson DF, Cheung CY. Fetal swallowing as a protective mechanism against oligohydramnios and polyhydramnios in late gestation sheep. Reprod Sci 20: 326–330, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brace RA, Cheung CY. Pre-delivery changes in amniotic fluid volume and composition in sheep. J Soc Gynecol Investig 12: 396–401, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Brace RA, Vermin ML, Huijssoon E. Regulation of amniotic fluid volume: intramembranous solute and volume fluxes in late gestation fetal sheep. Am J Obstet Gynecol 191: 837–846, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Dickson KA, Harding R. Decline in lung liquid volume and secretion rate during oligohydramnios in fetal sheep. J Appl Physiol 67: 2401–2407, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Faber JJ, Anderson DF. Regulatory response of intramembranous absorption and amniotic fluid to infusion of exogenous fluid in sheep. Am J Physiol Regul Integr Comp Physiol 277: R236–R242, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Faber JJ, Anderson DF. Absorption of amniotic fluid by amniochorion in sheep. Am J Physiol Heart Circ Physiol 282: H850–H854, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Faber J, Anderson D, Hohimer R, Yang Q, Giraud D, Davis L. Function curve of the membranes that regulate amniotic fluid volume in sheep. Am J Physiol Heart Circ Physiol 289: H146–H150, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Faber J, Brace RA, Davis LE, Anderson DF. Ovine amniotic fluid volume response to intra-amniotic balloon filling. Placenta 30: 201–202, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gagnon R, Harding R, Brace RA. Amniotic fluid and fetal urinary responses to severe placental insufficiency in sheep. Am J Obstet Gynecol 186: 1076–1084, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Gesteland KM, Anderson DF, Davis LE, Robertson P, Faber JJ, Brace RA. Intramembranous solute and water fluxes during high intramembranous absorption rates in fetal sheep with and without lung liquid diversion. Am J Obstet Gynecol 201: 85 e1–e6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert WM, Brace RA. Increase in fetal hydration during long-term intraamniotic saline infusion. Am J Obstet Gynecol 159: 1413–1417, 1988 [DOI] [PubMed] [Google Scholar]
- 14.Gilbert WM, Brace RA. The missing link in amniotic fluid volume regulation: intramembranous absorption. Obstet Gynecol 74: 748–754, 1989 [PubMed] [Google Scholar]
- 15.Jellyman JK, Cheung CY, Brace RA. Amniotic fluid volume responses to esophageal ligation in fetal sheep: contribution of lung liquid. Am J Obstet Gynecol 200: 313 e1–e313 e6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kullama LK, Agnew CL, Day L, Ervin MG, Ross MG. Ovine fetal swallowing and renal responses to oligohydramnios. Am J Physiol Regul Integr Comp Physiol 266: R972–R978, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Mann SE, Nijland MJ, Ross MG. Ovine adaptations to chronically reduced urine flow: preservation of amniotic fluid volume. J Appl Physiol 81: 2588–2594, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Moritz KM, Tangalakis K, Wintour EM. Renal, hormonal and cardiovascular responses to chronic angiotensin I infusion in the ovine fetus. Am J Physiol Regul Integr Comp Physiol 272: R1912–R1917, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Robertson P, Faber JJ, Brace RA, Louey S, Hohimer AR, Davis LE, Anderson DF. Responses of amniotic fluid volume and its four major flows to lung liquid diversion and amniotic infusions in the ovine fetus. Reproduc Sci 16: 88–93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross MG, Sherman DJ, Schreyer P, Ervin G, Day L, Humme J. Fetal rehydration via intraamniotic fluid: contribution of fetal swallowing. Pediatr Res 29: 214–217, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Scheve EJ, Brace RA. Amniotic fluid volume responses to intraamniotic infusion of lactate in fetal sheep. J Soc Gynecol Investig 7: 96–101, 2000 [PubMed] [Google Scholar]
- 22.Thurlow RW, Brace RA. Swallowing, urine flow, and amniotic fluid volume responses to prolonged hypoxia in the ovine fetus. Am J Obstet Gynecol 189: 601–608, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Tomoda S, Brace RA, Longo LD. Amniotic fluid volume and fetal swallowing rate in sheep. Am J Physiol Regul Integr Comp Physiol 249: R133–R138, 1985 [DOI] [PubMed] [Google Scholar]