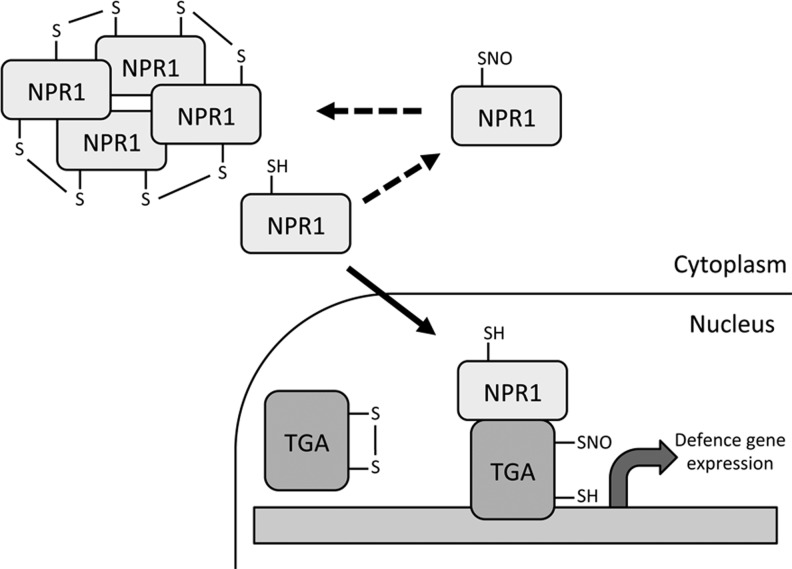
FIG. 5.
Redox-regulation of nonexpressor of pathogenesis-related genes 1 (NPR1) and TGA transcription factors. NPR1 exists as an oligomer in the cytoplasm, facilitated by intermolecular disulfide bonds. Upon pathogen attack these disulfides are reduced and monomeric NPR1 translocates to the nucleus where it interacts with TGA transcription factors and exerts its co-activator function on defense-related genes. S-nitrosylation of NPR1 promotes its oligomerization and thus inhibits its nuclear translocation. Formation of intramolecular disulfide bonds within TGAs prevent NPR1 binding, however, S-nitrosylation of TGAs may prevent these disulfides from forming, leading to increased defense gene expression.