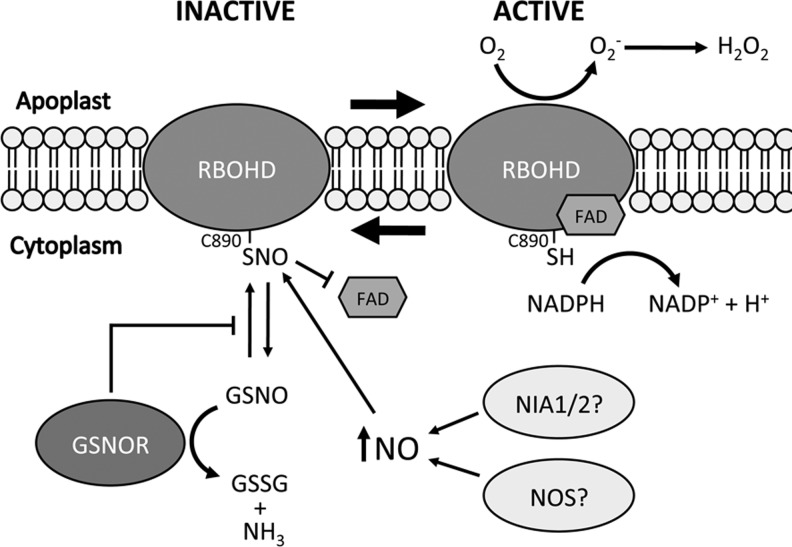
FIG. 8.
S-nitrosylation of RBOHD inhibits FAD binding and its NADPH oxidase activity. S-nitrosylation of RBOHD at Cys890 blunts its NADPH oxidase activity by inhibiting the binding of the cofactor FAD. This may serve as a mechanism to attenuate cell death during immune responses through negative feedback signaling by increasing levels of NO. In gsnor1-3 mutant plants, RBOHD activity is reduced presumably due to its increased S-nitrosylation.