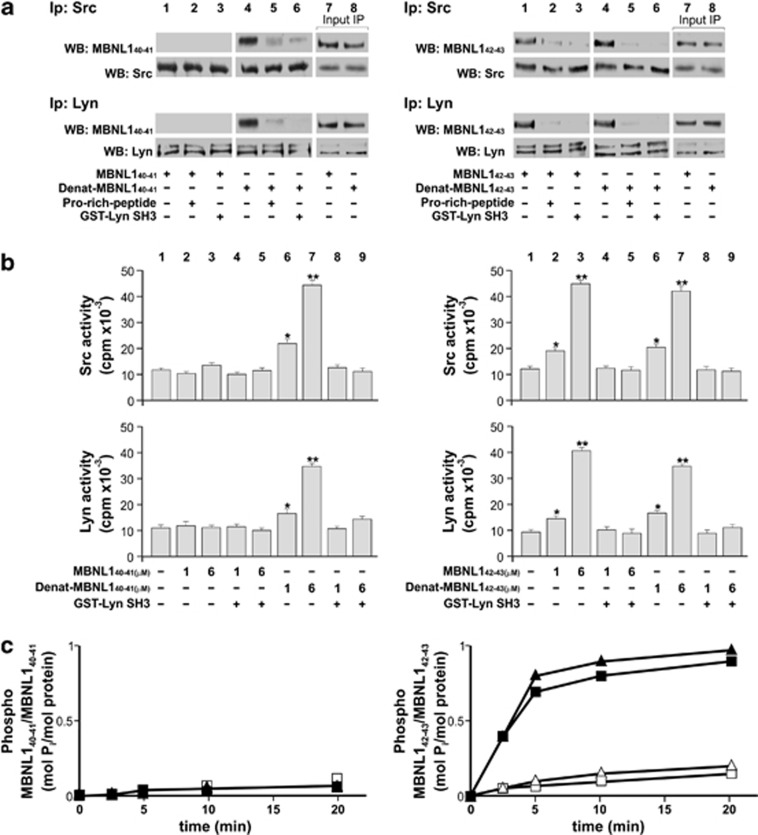
Figure 3.
Analysis of the interaction between the SH3 domain of the SFKs Src and Lyn and the proline-rich motif of recombinant MBNL140–41 and MBNL142–43 isoforms. (a) The interaction between SH3 domain of two SFK (Src and Lyn) and the proline-rich motifs of recombinant MBNL140–41 and MBNL142–43 isoforms. The 0.2 μg of recombinant MBNL140–41 or MBNL142–43 (as such or heated at 55 °C for 10 min, denat. MBNL1) was incubated with 0.1 μg of Src or Lyn in the absence or presence, respectively, of a proline-rich peptide or the GST-Lyn SH3 domain, for 10 min at 30 °C. Three-fourths of the sample underwent immunoprecipitation with anti-Src or anti-Lyn antibodies, respectively. Immunocomplexes were then probed with anti-P11 or anti-P9 antibodies, and subsequently with anti-Src or anti-Lyn antibodies. The presence of MBNL42 or MBNL43 (right panel), but not of MBNL40 or MBNL41 (left), indicates its association with the two SFKs and suggests that the stretch encoded by exon 5 might have a role in this interaction, possibly through a conformational change that renders the PRMs available to binding. Moreover, competition assays carried out in the absence or presence of a synthetic proline-rich peptide (5′-KGGRSRLPLPPLPPPG-3′) known to interact with the Lyn SH3 domain, or the GST-Lyn/SH3 domain, confirmed that this interaction was mediated by PRMs and the SH3 domain. The remainder of the sample underwent WB analysis (lanes 7 and 8) (b) As this interaction was also a prerequisite for enhancement of SFK activity relative to the basal state, we tested Tyr kinase activity of Src (top panel) and Lyn (bottom) on the Src-specific peptide substrate cdc2 in the absence (lane 1) or presence of increasing concentrations of MBNL141 (left panels, lanes 2–5) and denat. MBNL141 (left panels, lanes 6–9) or MBNL143 (right panels, lanes 2–5) and denat. MBNL143 (right panels, lanes 6–9), alternatively supplemented with the GST-Lyn SH3 domain (lanes 4 and 5, and 8–9) as described in the Materials and Methods section. (c) As this interaction was also a prerequisite for recognition of MBNL143 as a substrate by SFKs, we tested whether MBNL141 or MBNL143 (each 1 μM) were phosphorylated by Lyn and Src in the absence (solid triangles and solid squares, respectively) or presence (open triangles and open squares, respectively) of the GST-Lyn SH3 domain. At various time points, reactions were stopped and the phosphate incorporated was analyzed after SDS-PAGE on the Cyclone Plus. The amount is expressed as mol P incorporated/mol prot. (values represent the mean of three separate experiments±S.D.). As expected, only MBNL143 is phosphorylated by Src. The same set of experiments conducted with the MBNL140 and MBNL142 isoforms demonstrated that these two proteins behave similarly to MBNL141 and MBNL143, respectively (data not shown). Only MBNL142–43 isoforms were able to significantly increase Src and Lyn activity, suggesting a novel function of these long isoforms in kinase enzyme regulation. Data are expressed as mean±S.D. from three separate experiments. *P<0.05; ***P<0.001 by Kruskal–Wallis analysis