Fig. 5.
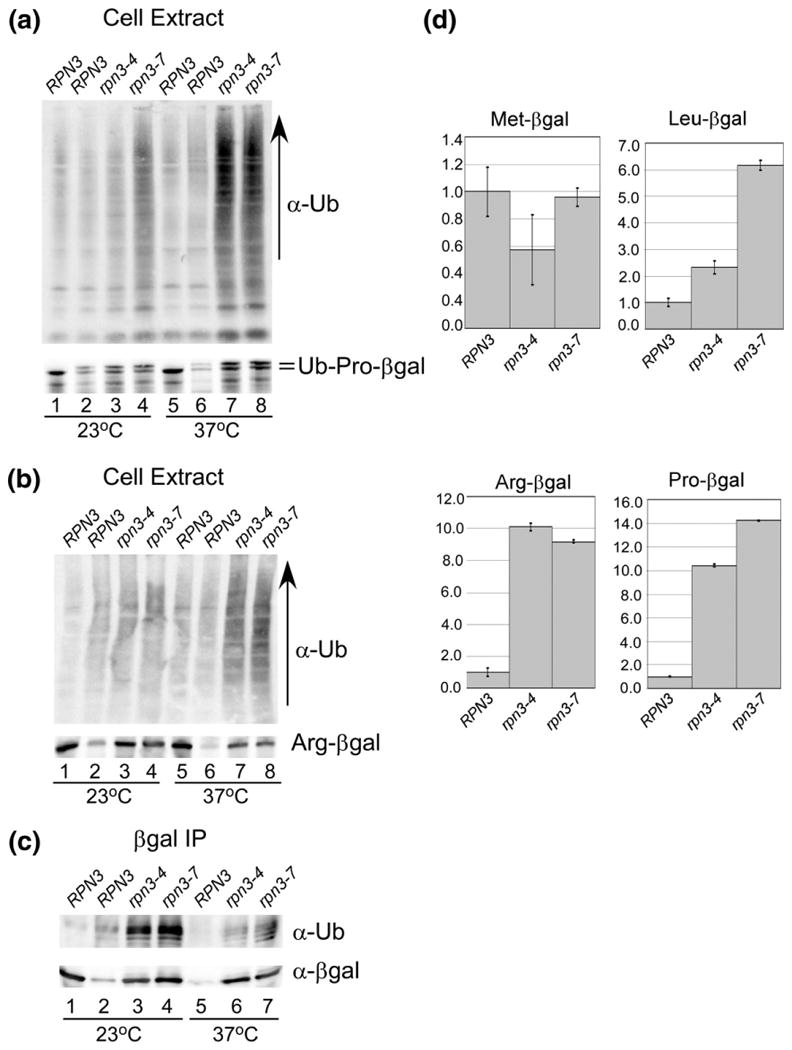
Defective proteolysis in rpn3 mutants. (a) Protein extracts were prepared from RPN3, rpn3-4, and rpn3-7 expressing Ub-Pro-βgal. RPN3 expressing a control protein (Met-βgal) is characterized in lane 1. An equal amount of protein extract was resolved by SDS/PAGE and examined by immunoblotting using antibodies against ubiquitin and βgal. (b) Similar to (a), protein extracts expressing Arg-βgal were characterized. RPN3 expressing a control protein (Met-βgal) is characterized in lane 1. An equal amount of protein extract was resolved by SDS/PAGE and examined by immunoblotting using antibodies against ubiquitin and βgal. (c) The extracts examined in (b) were incubated with βgal antibody to immunoprecipitate Arg-βgal, and the immunoprecipitated proteins were examined with antibodies against ubiquitin (upper panel) and βgal (lower panel). (d) Substrates Leu-βgal, Arg-βgal, and Ub-Pro-βgal were expressed in RPN3, rpn3-4, and rpn3-7, and β-galactosidase activity was measured in triplicate. The data were quantified and standardized to the expression of the protein in the wildtype strain, and plotted.
