Fig. 8.
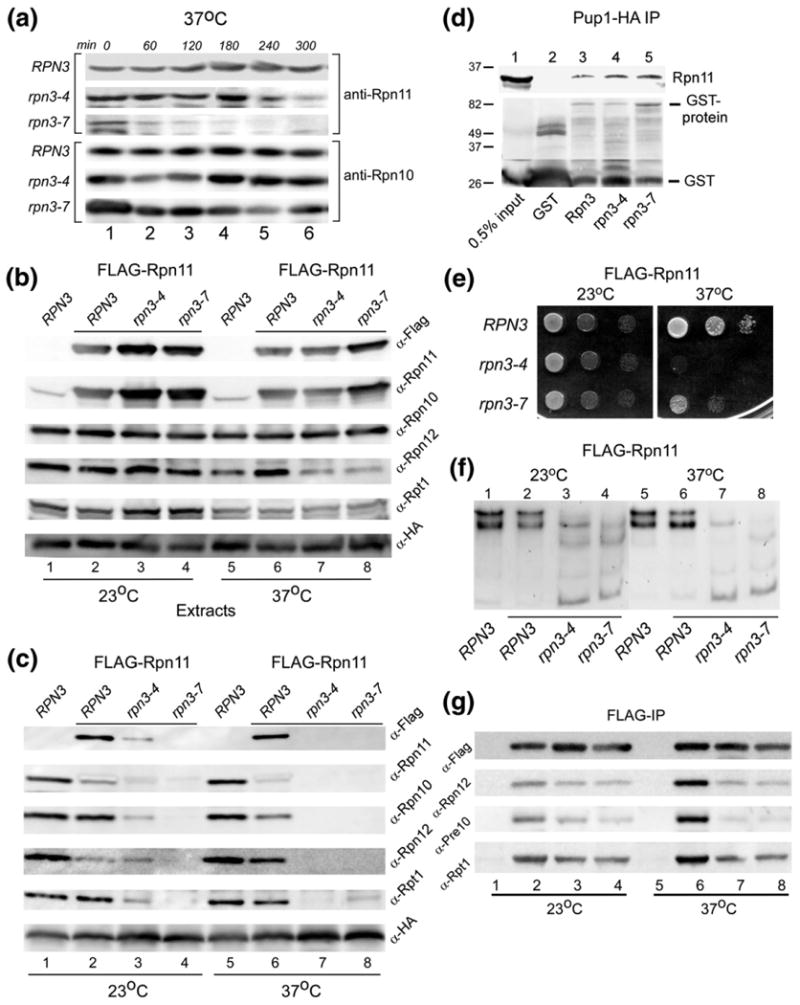
WT Rpn11 is destabilized in rpn3 mutants. (a) The stability of native Rpn11 was determined in RPN3, rpn3-4, and rpn3-7. Yeast cells were pre-grown at 23 °C and then transferred to 37 °C. Aliquots were withdrawn at the intervals shown, and the level of Rpn11 was determined by immunoblotting using anti-Rpn11 antibodies. The same filter was subsequently probed with antibodies against Rpn10 (lower panel). (b) RPN3, rpn3-4, and rpn3-7 were transformed with a high-copy plasmid expressing FLAG-Rpn11. Cultures were grown at 23 °C and then transferred to 37 °C for 3 h. An equal amount of total protein extract was examined by immunoblotting using antibodies indicated on the right. Lanes 1 and 5 represent control WT strain, lacking FLAG-Rpn11 overexpression. The level of both native Rpn11 and FLAG-Rpn11 was estimated by comparing the reaction with both anti-FLAG and anti-Rpn11 antibodies (compare lanes 1 and 2). (c) The extracts described in (a) were incubated with anti-HA antibody to immunoprecipitate (targeting Pup1-HA in the 20S core particle). The immunoprecipitates were resolved by SDS/PAGE and characterized using antibodies indicated on the right. (d) GST-Rpn3, GST-rpn3-4, and GST-rpn3-7 were bound to glutathione Sepharose, and their interaction with recombinant His6-Rpn11 was determined by immunoblotting. A control lane containing only GST is also shown (lane 2). (e) Yeast cells overexpressing FLAG-Rpn11 were spotted on agar medium (using serial 10-fold dilutions), and suppression of the rpn3 growth defect at 37 °C was investigated. (f) The protein extracts described in (b) were separated in a native polyacrylamide gel, and in situ hydrolysis of proteasome substrate LLVY-AMC was determined. (g) The protein extracts described in (b) were incubated with FLAG agarose to immunoprecipitate FLAG-Rpn11. The co-purification of 19S and 20S subunits was determined by immunoblotting.
