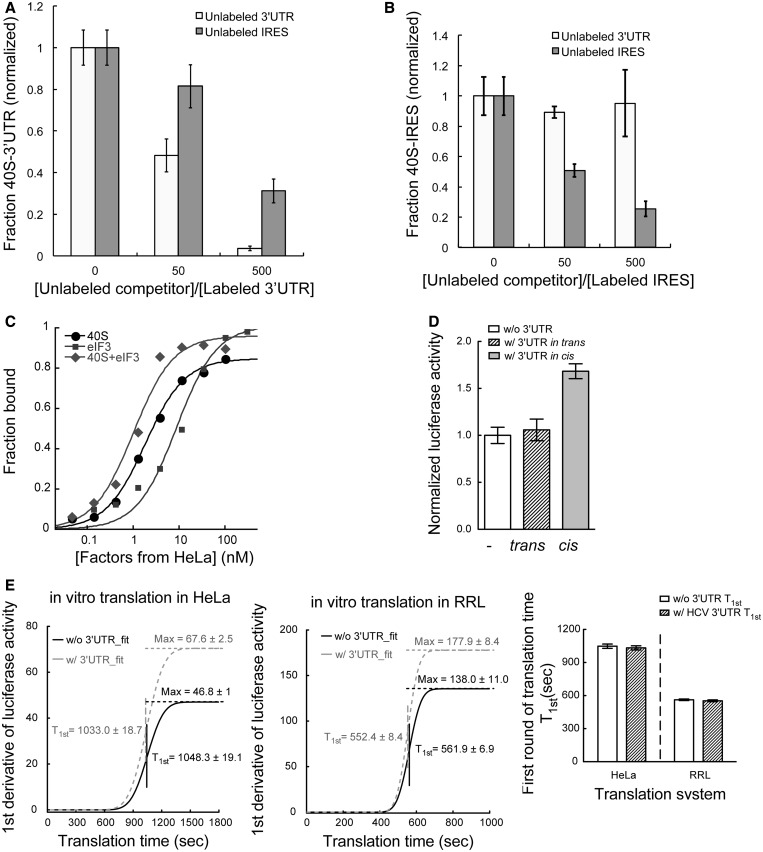
Figure 5.
The HCV 3′UTR stimulates translation only in cis, without affecting the rate of translation (A). Competition assaying showing the 3′UTR-40S interaction is subject to partial competition by unlabeled IRES (dark gray) with the competition less efficient than that from unlabeled 3′UTR (light gray). The competition by 50 times of unlabeled 3′UTR seems modest because the 40S concentration (10 nM) used in these experiment is well above Kd. (B). The IRES-40S interaction is subject to competition by unlabeled IRES (dark gray) but not unlabeled 3′UTR (light gray). The x-axis shows the ratio between unlabeled competitor and radiolabeled probe. (C). Binding isotherms for the interactions between the HCV 3′UTR and the 40S subunit (Kd = 1.8 ± 0.1 nM), eIF3 (Kd = 8.9 ± 2 nM), as well as the 40S-eIF3 complex (Kd = 1.0 ± 0.2 nM) (D). In vitro translation assays showing the 3′UTR can only stimulate translation when in cis with the IRES and reporter mRNA. Addition of free 3′UTR in trans showed no stimulation on IRES-dependent translation. (E). First derivative of the real-time recorded luciferase activity was fitted to a cumulative function of normal distribution. Values for both T1st and Max are labeled. For both RRL (left) and Hela lysate (middle) system, presence of the 3′UTR does not affect the T1st but leads to a higher Max value. The bar graph (right) shows the comparison of the T1st for transcripts with and without the HCV 3′UTR in both translation systems.